We serve Diosgenin CAS:512-04-9 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
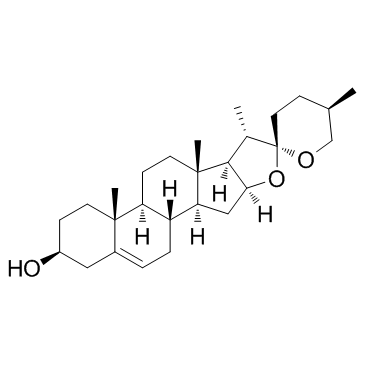
Contact us for information like Diosgenin chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,(3β,25R)-Spirost-5-en-3-ol physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Diosgenin Use and application,3β-Hydroxy-5-spirostene technical grade,usp/ep/jp grade.
Related News: At present, Teva can produce more than 300 generic drugs, and the API department has approximately 650 authorized patents and patent applications worldwide. It is also the generic drug company with the most challenges in patenting ParagraphIV in the world.4-(4-Bromopyrazol-1-yl)Piperidine-1-Carboxylic Acid Tert-Butyl Ester manufacturer You will find the name and amount of the active ingredient contained in the medicine on the package of OTC (over-the-counter) drugs.3-(Methylthio)butanal supplier The drug review center will establish a registration platform and database for APIs, pharmaceutical excipients and pharmaceutical packaging materials, which means that the domestic drug substance DMF system is expected to be gradually implemented.1,2-difluorobenzene vendor Others — somewhat more cynically — wondered if this was a concerted effort to promote certain herbal products to boost their makers’ share prices ahead of the Chinese stock market’s re-opening Monday.The clinical trial International Study of Phase 3 IV RigosErtib, or INSPIRE, was finalized following guidance received from the U.S. Food and Drug Administration and European Medicines Agency.

