We serve Tetramethylammonium fluoride tetrahydrate CAS:17787-40-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
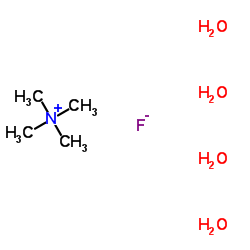
Contact us for information like Tetramethylammonium fluoride tetrahydrate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,TMAF physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Tetramethylammonium fluoride tetrahydrate Use and application,Tetramethylammonium fluoride tetrahydrate technical grade,usp/ep/jp grade.
Related News: The new restrictions on foreign nationals begin on February 3, Prime Minister Jacinda Ardern announced in a press release Sunday.(R)-3-(3-Fluoro-4-morpholinophenyl)-5-(hydroxymethyl)oxazolidin-2-one manufacturer GlaxoSmithKline’s HIV drugs division ViiV Healthcare said on Saturday that the U.S. Food and Drug Administration declined to approve its long acting HIV injection.3-(2-chloropyrimidin-4-yl)-1-methylindole supplier GlaxoSmithKline’s HIV drugs division ViiV Healthcare said on Saturday that the U.S. Food and Drug Administration declined to approve its long acting HIV injection.4-bromobutan-1-ol vendor Once they have decided how to make the compound, our staff in the production department manufacture a high quantity of APIs using the large reactors in our plant.Patents covering oral and injectable rigosertib have been issued in the US and are expected to provide coverage until at least 2037.

