We serve Chemical Name:2-cyclohexylethanimidamide,hydrochloride CAS:816469-55-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
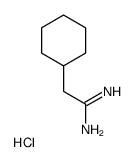
Chemical Name:2-cyclohexylethanimidamide,hydrochloride
CAS.NO:816469-55-3
Synonyms:2-cyclohexylethanimidamide hydrochloride;2-Cyclohexyl-acetamidine HCl
Molecular Formula:C8H17ClN2
Molecular Weight:176.68700
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:264.6ºC at 760 mmHg
Density:N/A
Index of Refraction:
PSA:49.87000
Exact Mass:176.10800
LogP:3.49480
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 2-cyclohexylethanimidamide hydrochloride chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-Cyclohexyl-acetamidine HCl physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-Cyclohexyl-acetamidine HCl Use and application,2-cyclohexylethanimidamide hydrochloride technical grade,usp/ep/jp grade.
Related News: China’s pharmaceutical CMO market has recently maintained a growth rate of more than 10%. From the perspective of market structure, the average growth rate of clinical production is 9.5%, while the average growth rate of commercial production will reach 18.7%. 2-cyclohexylethanimidamide,hydrochloride manufacturer There’s a few factors that play into this. 2-cyclohexylethanimidamide,hydrochloride supplier Factors that have a significant impact on the sales volume of APIs come from the production side, which mainly includes costs and processes. 2-cyclohexylethanimidamide,hydrochloride vendor Different cell types can be affected, although the most common finding in MDS is a shortage of red blood cells (anaemia). Patients with higher-risk MDS may progress to the development of acute leukaemia. 2-cyclohexylethanimidamide,hydrochloride factory In a statement to Fierce Medtech, Innova said it has completed some corrective actions, while others are still underway, and that the U.S. recall was launched to reclaim tests distributed to employees, clinical studies and to customers for early evaluation. The company said it plans to seek an emergency use authorization and comply with all FDA requirements.

