We serve Tetrapropyl Ammonium Fluoride CAS:7217-93-8 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
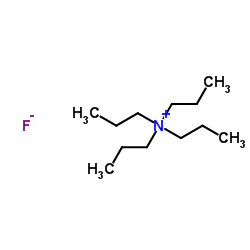
Contact us for information like Tetrapropyl Ammonium Fluoride chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,TPAF physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,N,N,N-Tripropyl-1-propanaminium fluoride Use and application,N,N,N-Tripropyl-1-propanaminium fluoride technical grade,usp/ep/jp grade.
Related News: That notice came two days after Chinese President Xi Jinping — a well known TCM advocate — called for the “combination of Chinese and Western medicine” in the diagnosis and treatment of the disease at a meeting of the Communist Party’s Politburo Standing Committee, the country’s supreme ruling body.3-Borono-5-fluorobenzoic acid manufacturer The intravenous form of rigosertib has been studied in Phase 1, 2, and 3 clinical trials involving more than 1000 patients, and is currently being evaluated in a randomized Phase 3 international INSPIRE trial for patients with HR-MDS after failure of HMA therapy.cis-4,7,10,13,16,19-Docosahexaenoic acid methyl ester supplier The intravenous form of rigosertib has been studied in Phase 1, 2, and 3 clinical trials involving more than 1000 patients, and is currently being evaluated in a randomized Phase 3 international INSPIRE trial for patients with HR-MDS after failure of HMA therapy.2,2',4,4'-Tetrahydroxybenzophenone vendor The move could force international drugmakers to further cut prices and enable copycat medicines to replace imported off-patent brands at faster pace.From a global perspective, China’s API companies have also performed well. According to the report of the American Transparent Medicine website, in 2016 the top ten pharmaceutical companies in the global API market, Chinese pharmaceutical companies accounted for 6 seats.

