We serve Growth hormone releasing peptide CAS:87616-84-0 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
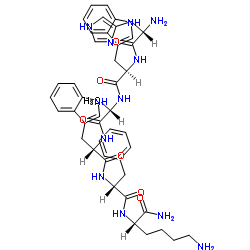
Contact us for information like Growth hormone releasing peptide chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,GH-Releasing hexapeptide 6 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,GH-Releasing hexapeptide 6 Use and application,Growth hormone releasing peptide technical grade,usp/ep/jp grade.
Related News: Under the terms of this agreement, Inceptua will support Onconova through the pre-approval provision of intravenous rigosertib initially into a number of countries including: Australia, Denmark, Finland, France, Ireland, Italy, the Netherlands, Portugal, South Africa, Spain, and the UK.Phenylacetic acid manufacturer The downstream industry of the pharmaceutical intermediate industry is mainly the production of APIs, and the relationship between APIs and preparations is in the upstream and downstream industry chain. The consumer demand for downstream preparations will directly affect the demand for APIs.3-Methyl-6-nitroindazole supplier DPH and BPHC continue to work closely with the CDC to maintain vigilance during this virus outbreak.2-Methyl-5-nitropyridine vendor Trial results demonstrated that Oligomannate statistically improved cognitive function in mild-to-moderate AD patients as early as week 4 and the benefit was sustained at each follow-up assessment visit,” Shanghai Green Valley Pharmaceuticals, which developed the drug along with two academic institutions in China, said in a statement.Trial results demonstrated that Oligomannate statistically improved cognitive function in mild-to-moderate AD patients as early as week 4 and the benefit was sustained at each follow-up assessment visit,” Shanghai Green Valley Pharmaceuticals, which developed the drug along with two academic institutions in China, said in a statement.

