We serve Icatibant Acetate CAS:30308-48-4 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
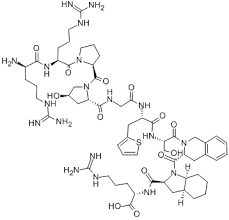
Contact us for information like Icatibant Acetate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Icatibant physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Icatibant Acetate Use and application,Icatibant technical grade,usp/ep/jp grade.
Related News: It is estimated that the size of China’s CMO market has reached about USD 5 billion. With the implementation and further implementation of the MAH system (the core is to separate drug marketing authorization from drug production authorization) in China, it is expected to promote the outbreak of the domestic drug CMO industry. It will grow at a rate of 20% to 30%, of which patented APIs occupy a larger proportion.Trichloroacetaldehyde manufacturer ICIG companies currently employ more than 3,000 people and operate 15 manufacturing facilities in Europe and the United States.(4S,5R)-2,4-Diphenyl-4,5-dihydrooxazole-5-carboxylic acid supplier ICIG companies currently employ more than 3,000 people and operate 15 manufacturing facilities in Europe and the United States.1,1'-Biphenyl]-4,4'-diamine, N,N'-bis(4-aminophenyl)-N,N'-diphenyl- vendor The government is leveraging its large patient population to push non-domestic drugs companies to cut prices to their lowest level globally.The foundational patent, which expires in 2034, is owned by MSK and is licensed exclusively to Fate Therapeutics for all human therapeutic uses.

