We serve 2-(Heptafluoropropoxy)hexafluoropropyl Trifluorovinyl Ether CAS:1644-11-7 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
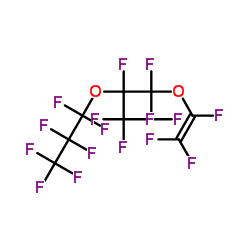
Contact us for information like 2-(Heptafluoropropoxy)hexafluoropropyl Trifluorovinyl Ether chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-(perfluoropropoxy)perfluoropropyl trifluorovinyl ether physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-(Heptafluoropropoxy)hexafluoropropyl Trifluorovinyl Ether Use and application,2-(Heptafluoropropoxy)hexafluoropropyl Trifluorovinyl Ether technical grade,usp/ep/jp grade.
Related News: From the perspective of the corresponding formulation manufacturer, the drug substance needs to meet the requirements of impurities and stability. The production base must pass the international quality system certifications such as cGMP and EuGMP. At the same time, the drug substance company must have sufficient capacity.(1S,2S)-(1-benzyl-3-chloro-2-hydroxypropyl)carbamic acid tert-butyl ester manufacturer From the perspective of the corresponding formulation manufacturer, the drug substance needs to meet the requirements of impurities and stability. The production base must pass the international quality system certifications such as cGMP and EuGMP. At the same time, the drug substance company must have sufficient capacity.Vitamin A supplier Here’s what Apple told CNN Business in a statement:hexyl isocyanate vendor It is frequently associated with the presence of blasts or leukemic cells in the marrow.It is frequently associated with the presence of blasts or leukemic cells in the marrow.

