We serve Antioxidant DPIOP CAS:26401-27-4 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
| TEST | UNIT | SPECIFICATION |
| APPEARANCE | COLORLESS LIQUID | |
| REFRACTIVE INDEX | 1.5200-1.5300 | |
| SPECIFIC GRAVITY | 1.040-1.050 |
PRODUCT FEATURES AND APPLICATIONS
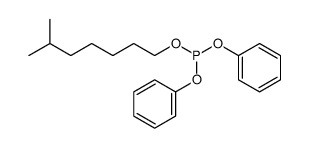
● DPIOP is high molecule alkyl-aryl phosphite ester
antioxidants,with very high phosphorus
● DPIOP has stronger ability to decompose hydroperoxide(ROOH)
than common phosphiteAntioxidants.
● DPIOP is widely used as secondary antioxidant in many polymers including PVC Polyurethane(PU) and etc, it can be used with hinder phenolic antioxidant and UV Absorbers to have Synergist effect.
-PVC Film
-Polyurethane Film and glue
-Rubber and Latex
-Rosin and unsaturated resins
-PUR Coatings
PACKAGE
200 KG Drum
HANDLING AND STORAGE
Keep container tightly closed and dry and storage in cool place
Contact us for information like Antioxidant DPIOP chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Diphenyl isooctyl phosphite physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,isooctyl diphenyl phosphite Use and application,Phosphorous acid 6-methylheptyldiphenyl ester technical grade,usp/ep/jp grade.
Related News: “Inceptua Medicines Access is delighted to be selected as Onconova’s partner for the Pre-approval Access Program for rigosertib.2,4-bis-acetylamino-5-(3,4-dimethoxy-benzyl)-pyrimidine manufacturer “Inceptua Medicines Access is delighted to be selected as Onconova’s partner for the Pre-approval Access Program for rigosertib.Allyl-phosphonic acid 2-bromo-ethyl ester 2-chloro-ethyl ester supplier Their pharmacological activity plays a direct role in the diagnosis, cure, mitigation, treatment, or prevention of disease.L-Proline, glycyl-N6-(2-methylpropyl)-L-lysyl-, monoacetate vendor From the perspective of the overall life cycle of generic drugs from R & D to sales, with the expiration of patents, the business model for the production and marketing of generic pharmaceutical raw materials has changed, demand has increased, business potential has been realized quickly, and gross profit margins have shown a downward trend.From the perspective of the overall life cycle of generic drugs from R & D to sales, with the expiration of patents, the business model for the production and marketing of generic pharmaceutical raw materials has changed, demand has increased, business potential has been realized quickly, and gross profit margins have shown a downward trend.

