We serve Chemical Name:4-bromo-7-methylisatin CAS:874375-17-4 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
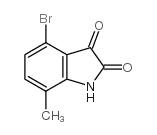
Chemical Name:4-bromo-7-methylisatin
CAS.NO:874375-17-4
Synonyms:4-Bromo-7-methylisatin;4-Bromo-7-methylindoline-2,3-dione;MFCD06659469;4-bromo-7-methylisatine
Molecular Formula:C9H6BrNO2
Molecular Weight:240.05300
HS Code:2933990090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:1.714g/cm3
Index of Refraction:1.631
PSA:46.17000
Exact Mass:238.95800
LogP:2.03030
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 4-Bromo-7-methylisatin chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,4-bromo-7-methylisatine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,MFCD06659469 Use and application,4-Bromo-7-methylindoline-2,3-dione technical grade,usp/ep/jp grade.
Related News: Pre-approval Access Programs (also known as expanded access, early access, compassionate use, named patient supply) are regulatory-compliant processes permitting experimental agents in development to be made available upon the request of a physician or a patient for appropriate patients for whom no alternative treatment option exists in their country. 4-bromo-7-methylisatin manufacturer ��No two studies are the same, and each carries its own unique risks and presents individual challenges, but the aim of CTSuccess is to use the experience we have as a company in supplying materials for thousands of trials to identify the characteristics of a project upfront and highlight potential risk factors that may arise,�� said Kristen Devito, Global Director, Catalent Clinical Supply Services. 4-bromo-7-methylisatin supplier The Darzalex-Rd regimen got its FDA go-ahead in transplant-ineligible patients in 2019 based on data from the same phase 3 MAIA trial showing it could pare down the risk of disease progression or death by 44% after a median follow-up of 28 months. Now, after 56.2 months of follow-up, over half of Darzalex patients were still alive without their disease worsening, a showing that’s again “unprecedented” for a front-line myeloma treatment, Tendler said. 4-bromo-7-methylisatin vendor From the perspective of product structure, the varieties of these enterprises are mainly concentrated in vitamins, antipyretics and analgesics, antibiotics and corticosteroids (ie bulk drug substances). 4-bromo-7-methylisatin factory Pre-approval Access Programs (also known as expanded access, early access, compassionate use, named patient supply) are regulatory-compliant processes permitting experimental agents in development to be made available upon the request of a physician or a patient for appropriate patients for whom no alternative treatment option exists in their country.

