We serve Chemical Name:(2S)-Amino(3-fluorophenyl)acetic acid CAS:154006-66-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
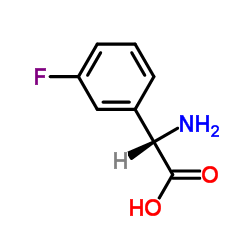
Chemical Name:(2S)-Amino(3-fluorophenyl)acetic acid
CAS.NO:154006-66-3
Synonyms:(2S)-Amino(3-fluorophenyl)acetic acid;3-Fluorophenylglycine;Benzeneacetic acid, α-amino-3-fluoro-, (αS)-
Molecular Formula:C8H8FNO2
Molecular Weight:169.153
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:286.5±30.0 °C at 760 mmHg
Density:1.3±0.1 g/cm3
Index of Refraction:1.565
PSA:63.32000
Exact Mass:169.053909
LogP:0.99
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like (2S)-Amino(3-fluorophenyl)acetic acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Benzeneacetic acid, α-amino-3-fluoro-, (αS)- physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,(2S)-Amino(3-fluorophenyl)acetic acid Use and application,(2S)-Amino(3-fluorophenyl)acetic acid technical grade,usp/ep/jp grade.
Related News: Therefore, the growth of the global pharmaceutical market has greatly affected the development of the entire intermediate and API industry. (2S)-Amino(3-fluorophenyl)acetic acid manufacturer The StartScore model was developed by the company��s in-house supply chain experts based on actual performance data. (2S)-Amino(3-fluorophenyl)acetic acid supplier The Big Pharma had, however, decided to all but shelve donanemab in the near term as it couldn’t see a regulatory path forward. (2S)-Amino(3-fluorophenyl)acetic acid vendor Therefore, the growth of the global pharmaceutical market has greatly affected the development of the entire intermediate and API industry. (2S)-Amino(3-fluorophenyl)acetic acid factory The Darzalex-Rd regimen got its FDA go-ahead in transplant-ineligible patients in 2019 based on data from the same phase 3 MAIA trial showing it could pare down the risk of disease progression or death by 44% after a median follow-up of 28 months. Now, after 56.2 months of follow-up, over half of Darzalex patients were still alive without their disease worsening, a showing that’s again “unprecedented” for a front-line myeloma treatment, Tendler said.

