We serve Chemical Name:3-bromo-4-ethoxybut-3-en-2-one CAS:82982-59-0 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
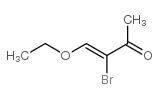
Chemical Name:3-bromo-4-ethoxybut-3-en-2-one
CAS.NO:82982-59-0
Synonyms:3-BROMO-4-ETHOXY-3-BUTEN-2-ONE;3-Buten-2-one,3-bromo-4-ethoxy
Molecular Formula:C6H9BrO2
Molecular Weight:193.03800
HS Code:2914700090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:243.9ºC at 760 mmHg
Density:1.402g/cm3
Index of Refraction:1.482
PSA:26.30000
Exact Mass:191.97900
LogP:1.84820
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 3-BROMO-4-ETHOXY-3-BUTEN-2-ONE chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,3-Buten-2-one,3-bromo-4-ethoxy physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,3-BROMO-4-ETHOXY-3-BUTEN-2-ONE Use and application,3-Buten-2-one,3-bromo-4-ethoxy technical grade,usp/ep/jp grade.
Related News: With the improvement of domestic GMP management level, the increase of process development capabilities and international certification experience, China has already possessed the conditions for developing characteristic APIs. 3-bromo-4-ethoxybut-3-en-2-one manufacturer Over the years, large international pharmaceutical companies have gradually focused on the research and development and sales of patented drugs, and have gradually outsourced traditional products. Among them, most of the contractors are pharmaceutical companies in emerging countries. 3-bromo-4-ethoxybut-3-en-2-one supplier For example, an active ingredient to relieve pain is included in a painkiller. 3-bromo-4-ethoxybut-3-en-2-one vendor For example, an active ingredient to relieve pain is included in a painkiller. 3-bromo-4-ethoxybut-3-en-2-one factory The Darzalex-Rd regimen got its FDA go-ahead in transplant-ineligible patients in 2019 based on data from the same phase 3 MAIA trial showing it could pare down the risk of disease progression or death by 44% after a median follow-up of 28 months. Now, after 56.2 months of follow-up, over half of Darzalex patients were still alive without their disease worsening, a showing that’s again “unprecedented” for a front-line myeloma treatment, Tendler said.

