We serve Chemical Name:Deterelix CAS:89662-30-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
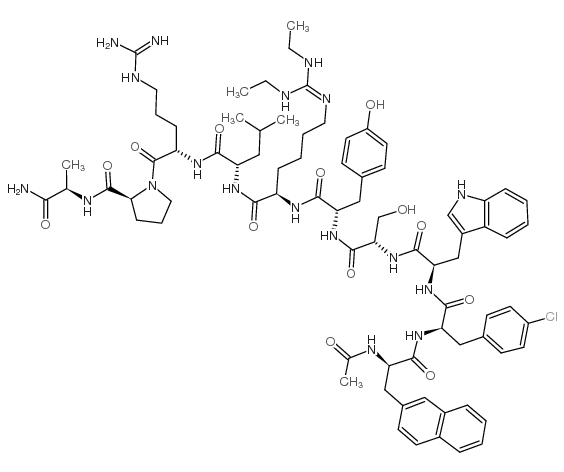
Chemical Name:Deterelix
CAS.NO:89662-30-6
Synonyms:Deterelix
Molecular Formula:C78H105ClN18O13
Molecular Weight:1538.23000
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:1.658
PSA:479.87000
Exact Mass:1536.78000
LogP:8.00600
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like Deterelix chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Deterelix physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Deterelix Use and application,Deterelix technical grade,usp/ep/jp grade.
Related News: DMF is the main management method for APIs in developed countries in Europe and the United States. Under the DMF system, API companies can submit DMF filing documents to the regulatory authority at any time, but the regulatory authority will not conduct technical reviews on them. When the drug is administered, the regulatory authority will associate and review the drug substance and the preparation. Deterelix manufacturer It is foreseeable that in the future, the capacity and output of bulk APIs in China will decrease, the supply-demand relationship will be balanced, and prices and profits will gradually return to a more reasonable range. The era of low prices in the past will be gone forever. Individual APIs Varieties may even lose their price competitive advantage and move abroad. Deterelix supplier We partner with life science companies of all sizes, drawing on over 20 years of industry experience. Deterelix vendor DMF is the main management method for APIs in developed countries in Europe and the United States. Under the DMF system, API companies can submit DMF filing documents to the regulatory authority at any time, but the regulatory authority will not conduct technical reviews on them. When the drug is administered, the regulatory authority will associate and review the drug substance and the preparation. Deterelix factory The move follows a Class I recall, the FDA’s most serious, launched by Innova in late April amid “significant concerns” about the test’s accuracy—and alongside an official warning letter delivered to the company this week.

