We serve Chemical Name:(+)-endo-2-Aminonorbornane CAS:121055-07-0 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
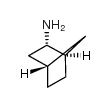
Chemical Name:(+)-endo-2-Aminonorbornane
CAS.NO:121055-07-0
Synonyms:exo-2-aminonorbornane
Molecular Formula:C7H13N
Molecular Weight:111.18500
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:160ºC
Density:0.985
Index of Refraction:
PSA:26.02000
Exact Mass:111.10500
LogP:1.83400
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like exo-2-aminonorbornane chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,exo-2-aminonorbornane physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,exo-2-aminonorbornane Use and application,exo-2-aminonorbornane technical grade,usp/ep/jp grade.
Related News: Both patients were being treated in isolation units at a Manila hospital. (+)-endo-2-Aminonorbornane manufacturer Additional results from the trial with data on another goal of extending survival is expected in 2020, AstraZeneca said. (+)-endo-2-Aminonorbornane supplier As one of the world’s largest API producers and exporters, China’s chemical API production and operating income show steady growth. (+)-endo-2-Aminonorbornane vendor As a result, the Company��s platform is uniquely capable of overcoming numerous limitations associated with the production of cell therapies using patient- or donor-sourced cells, which is logistically complex and expensive and is subject to batch-to-batch and cell-to-cell variability that can affect clinical safety and efficacy. (+)-endo-2-Aminonorbornane factory As a result, the Company��s platform is uniquely capable of overcoming numerous limitations associated with the production of cell therapies using patient- or donor-sourced cells, which is logistically complex and expensive and is subject to batch-to-batch and cell-to-cell variability that can affect clinical safety and efficacy.

