We serve Chemical Name:2,2′-Furoin CAS:552-86-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
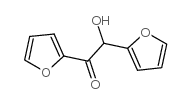
Chemical Name:2,2′-Furoin
CAS.NO:552-86-3
Synonyms:EINECS 209-024-8;1,2-Di-2-furanyl-2-hydroxyethanone;FUROIN;Ethanone,1,2-di-2-furanyl-2-hydroxy;2,2′-Furoin;Furoylfurylcarbinol;2,2′-DIPYRIDINYL DISULFIDE;MFCD00003246
Molecular Formula:C10H8O4
Molecular Weight:192.16800
HS Code:2932190090
Physical and Chemical Properties:
Melting point:134-137 °C(lit.)
Boiling point:306.8ºC at 760 mmHg
Density:1.32 g/cm3
Index of Refraction:1.557
PSA:63.58000
Exact Mass:192.04200
LogP:1.78890
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:NONH for all modes of transpor
Packing Group:
Contact us for information like EINECS 209-024-8 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,MFCD00003246 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,1,2-Di-2-furanyl-2-hydroxyethanone Use and application,EINECS 209-024-8 technical grade,usp/ep/jp grade.
Related News: Steven M. Fruchtman, M.D., President and Chief Executive Officer of Onconova, added ��The rigosertib Pre-approval Access Program is a key strategic initiative for Onconova. 2,2′-Furoin manufacturer In addition, although the API and intermediate industries are capital-intensive and technology-intensive industries, new competitors are still joining. 2,2′-Furoin supplier The government is leveraging its large patient population to push non-domestic drugs companies to cut prices to their lowest level globally. 2,2′-Furoin vendor Steven M. Fruchtman, M.D., President and Chief Executive Officer of Onconova, added ��The rigosertib Pre-approval Access Program is a key strategic initiative for Onconova. 2,2′-Furoin factory INSPIRE is a global, multi-center, randomized, controlled study to assess the efficacy and safety of IV rigosertib in higher-risk MDS (HR-MDS) patients who had progressed on, failed to respond to, or relapsed after previous treatment with a hypomethylating agent (HMA) within nine cycles over the course of one year after initiation of HMA treatment.

