We serve Chemical Name:1h-benzimidazole-2-sulfonic acid CAS:40828-54-4 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
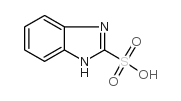
Chemical Name:1h-benzimidazole-2-sulfonic acid
CAS.NO:40828-54-4
Synonyms:1H-benzimidazol-2-sulphonic acid;1H-Benzoimidazole-2-sulfonic acid;Benzimidazol-2-ylsulfonic acid;MFCD00100972;benzimidazole-2-sulfonic acid;1H-Benzimidazole-2-sulfonicacid;Rabeprazole Impurity 20
Molecular Formula:C7H6N2O3S
Molecular Weight:198.19900
HS Code:2933990090
Physical and Chemical Properties:
Melting point:116-119 °C(lit.)
Boiling point:N/A
Density:1.681g/cm3
Index of Refraction:1.716
PSA:91.43000
Exact Mass:198.01000
LogP:1.89040
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:UN 3261 8/PG 2
Packing Group:
Contact us for information like 1H-benzimidazol-2-sulphonic acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Rabeprazole Impurity 20 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,benzimidazole-2-sulfonic acid Use and application,benzimidazole-2-sulfonic acid technical grade,usp/ep/jp grade.
Related News: The Philippines, the United States and Australia have expanded travel restrictions, temporarily barring noncitizens who have recently traveled to China. 1h-benzimidazole-2-sulfonic acid manufacturer This is how an API becomes a medicine. 1h-benzimidazole-2-sulfonic acid supplier In addition, the FDA approval ignored the recommendation of its outside advisors, who said Biogen did not provide enough evidence of clinical benefit. Three of the advisory panel’s members have resigned in protest since the FDA decision was announced on Monday. 1h-benzimidazole-2-sulfonic acid vendor The Philippines, the United States and Australia have expanded travel restrictions, temporarily barring noncitizens who have recently traveled to China. 1h-benzimidazole-2-sulfonic acid factory Exceeding impurities in the drug substance may cause the product and its preparation to be recalled. The company receives an FDA warning letter or a CEP certificate suspension, which in turn will cause customer compensation, product recall costs, and asset impairment losses to affect the company’s performance.

