We serve Chemical Name:L-Pyroglutamic Acid β-Naphthylamide CAS:22155-91-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
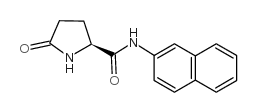
Chemical Name:L-Pyroglutamic Acid β-Naphthylamide
CAS.NO:22155-91-5
Synonyms:EINECS 244-809-9;L-Pyroglutamic acid 2-naphthylamide;L-PYROGLUTAMIC ACID β-NAPHTHYLAMIDE;MFCD00055911
Molecular Formula:C15H14N2O2
Molecular Weight:254.28400
HS Code:2933790090
Physical and Chemical Properties:
Melting point:188-193°C
Boiling point:616.9ºC at 760 mmHg
Density:1.311 g/cm3
Index of Refraction:1.682
PSA:58.20000
Exact Mass:254.10600
LogP:2.45870
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:UN 3077 9 / PGIII
Packing Group:
Contact us for information like EINECS 244-809-9 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,MFCD00055911 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,L-PYROGLUTAMIC ACID β-NAPHTHYLAMIDE Use and application,L-PYROGLUTAMIC ACID β-NAPHTHYLAMIDE technical grade,usp/ep/jp grade.
Related News: f you’ve traveled in or transited through mainland China, you won’t be allowed into New Zealand unless you’re a New Zealand national. L-Pyroglutamic Acid β-Naphthylamide manufacturer f you’ve traveled in or transited through mainland China, you won’t be allowed into New Zealand unless you’re a New Zealand national. L-Pyroglutamic Acid β-Naphthylamide supplier That’s four days earlier than it had initially planned. Delta’s last China-bound flight left on Saturday, February 1, and its final returning flight from China to the United States leaves on Sunday. L-Pyroglutamic Acid β-Naphthylamide vendor The national airlines of Egypt, Qatar and Saudi Arabia have halted flights to and from China. L-Pyroglutamic Acid β-Naphthylamide factory Exceeding impurities in the drug substance may cause the product and its preparation to be recalled. The company receives an FDA warning letter or a CEP certificate suspension, which in turn will cause customer compensation, product recall costs, and asset impairment losses to affect the company’s performance.

