We serve Chemical Name:Boc-(R)-3-amino-4-(4-chlorophenyl)-butyric acid CAS:218608-96-9 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
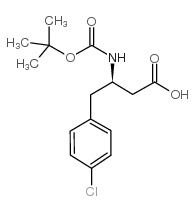
Chemical Name:Boc-(R)-3-amino-4-(4-chlorophenyl)-butyric acid
CAS.NO:218608-96-9
Synonyms:boc-(r)-3-amino-4-(4-chloro-phenyl)-butyric acid;MFCD01076276
Molecular Formula:C15H20ClNO4
Molecular Weight:313.77700
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:155 °C20 mm Hg(lit.)
Density:0.975 g/mL at 25 °C(lit.)
Index of Refraction:n20/D 1.516(lit.)
PSA:75.63000
Exact Mass:313.10800
LogP:3.64140
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:NONH for all modes of transpor
Packing Group:
Contact us for information like boc-(r)-3-amino-4-(4-chloro-phenyl)-butyric acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,MFCD01076276 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,MFCD01076276 Use and application,MFCD01076276 technical grade,usp/ep/jp grade.
Related News: The intravenous form of rigosertib has been studied in Phase 1, 2, and 3 clinical trials involving more than 1000 patients, and is currently being evaluated in a randomized Phase 3 international INSPIRE trial for patients with HR-MDS after failure of HMA therapy. Boc-(R)-3-amino-4-(4-chlorophenyl)-butyric acid manufacturer China’s pharmaceutical CMO market has recently maintained a growth rate of more than 10%. From the perspective of market structure, the average growth rate of clinical production is 9.5%, while the average growth rate of commercial production will reach 18.7%. Boc-(R)-3-amino-4-(4-chlorophenyl)-butyric acid supplier We also have to consider the degree of concentration and which temperature allows a high quality of API to be manufactured efficiently. Boc-(R)-3-amino-4-(4-chlorophenyl)-butyric acid vendor The intravenous form of rigosertib has been studied in Phase 1, 2, and 3 clinical trials involving more than 1000 patients, and is currently being evaluated in a randomized Phase 3 international INSPIRE trial for patients with HR-MDS after failure of HMA therapy. Boc-(R)-3-amino-4-(4-chlorophenyl)-butyric acid factory Aduhelm, however, is in a different league in terms of the number of potential patients and cost to the healthcare system.

