We serve Dititanium trioxide CAS:1344-54-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
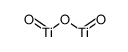
| Items of Analysis | Standard of Analysis | Test Results |
| Purity | ≥99.99% | 99.99% |
| Fe | <10ppm | 8ppm |
| Pb | <4ppm | 3ppm |
| Al | <3ppm | 2ppm |
| Cu | <3ppm | 1ppm |
| Ni | <4ppm | 2ppm |
| Sn | <3ppm | 2ppm |
| Mn | <2ppm | 1ppm |
| Cr | <3ppm | 2ppm |
| Si | <12ppm | 10ppm |
| Grain size | 200mesh | 200mesh |
| Conclusion | Conforms to Factory Standard | |
Contact us for information like Dititanium trioxide chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Titanium(III) oxide physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Titanium(III) oxide Use and application,Dititanium trioxide technical grade,usp/ep/jp grade.
Related News: Delta moved the date up after the US State Department warned that people should not travel to China due to concerns about the spread of coronavirus, which was first discovered in Wuhan, Hubei province, in December.1-Benzylpiperidin-3-one hydrochloride manufacturer The monthly injection to suppress the virus that causes AIDS was aimed as an alternative to daily pills.2-Bromo-4'-nitroacetophenone supplier The monthly injection to suppress the virus that causes AIDS was aimed as an alternative to daily pills.5-(N-t-Butoxycarbonyl-N-methyl)amino-2-p-methoxybenzylthiopentanonitrile vendor The monthly injection to suppress the virus that causes AIDS was aimed as an alternative to daily pills.DMF is the main management method for APIs in developed countries in Europe and the United States. Under the DMF system, API companies can submit DMF filing documents to the regulatory authority at any time, but the regulatory authority will not conduct technical reviews on them. When the drug is administered, the regulatory authority will associate and review the drug substance and the preparation.

