We serve 2-Bromo-6-chlorobenzaldehyde CAS:64622-16-8 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
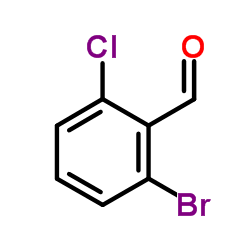
Contact us for information like 2-Bromo-6-chlorobenzaldehyde chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-bromo-6-chlorobenzaldehyde physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-Bromo-6-chlorobenzaldehyde Use and application,2-Bromo-6-chlorobenzaldehyde technical grade,usp/ep/jp grade.
Related News: Coupled with the poor global pesticide industry situation this year, the weakening of downstream customer demand has affected the sales of pesticide intermediates.Isobutylboronic Acid manufacturer GlaxoSmithKline’s HIV drugs division ViiV Healthcare said on Saturday that the U.S. Food and Drug Administration declined to approve its long acting HIV injection.Triethoxyvinylsilane supplier A Phase 1/2 trial of the combination therapy has been fully enrolled, and the preliminary efficacy and safety data was presented at The American Society of Hematology (ASH) Annual Meeting in December 2018.Diisopropyl malonate vendor “Out of an abundance of caution and based on the latest advice from leading health experts, we’re closing all our corporate offices, stores and contact centers in mainland China through February 9.”After several years of R & D and improvement, the production process of commonly used generic drug bulk drugs is relatively mature, and the products of similar companies have high similarities. Therefore, the competitive advantage of bulk drug companies is mainly reflected in cost control. Companies with cost advantages can usually pass Competition to expand production capacity and further gain scale advantages, while having stable, high-quality upstream supply.

