We serve Chemical Name:2-bromo-3-methoxybutanoic acid CAS:67819-23-2 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
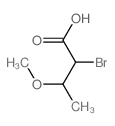
Chemical Name:2-bromo-3-methoxybutanoic acid
CAS.NO:67819-23-2
Synonyms:(+-)-erythro-2-bromo-3-methoxy-butyric acid;(+-)-erythro-2-Brom-3-methoxy-buttersaeure;2-bromo-3-methoxy-butyric acid;Butanoic acid,2-bromo-3-methoxy
Molecular Formula:C5H9BrO3
Molecular Weight:197.02700
HS Code:2918990090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:253.9ºC at 760 mmHg
Density:1.563g/cm3
Index of Refraction:1.485
PSA:46.53000
Exact Mass:195.97400
LogP:0.86940
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like (+-)-erythro-2-bromo-3-methoxy-butyric acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Butanoic acid,2-bromo-3-methoxy physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Butanoic acid,2-bromo-3-methoxy Use and application,2-bromo-3-methoxy-butyric acid technical grade,usp/ep/jp grade.
Related News: Beta Bionics is committed to obtaining regulatory approval and commercializing all three iLet configurations. 2-bromo-3-methoxybutanoic acid manufacturer The data will be updated at the upcoming 2019 ASH Annual Meeting next week. 2-bromo-3-methoxybutanoic acid supplier Under the sub-licensing agreement, Mankind will market the drug under its own trademark while Glenmark will manufacture and supply it to the drug firm. 2-bromo-3-methoxybutanoic acid vendor Beta Bionics is committed to obtaining regulatory approval and commercializing all three iLet configurations. 2-bromo-3-methoxybutanoic acid factory Biogen last month revived its plans to seek U.S. approval for its aducanumab treatment after announcing in March that it would terminate two large clinical trials for the drug. But some analysts believed FDA approval is highly unlikely.

