We serve Chemical Name:1,4,7,10-tetrathiacyclododecane CAS:25423-56-7 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
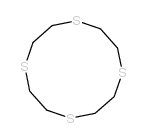
Chemical Name:1,4,7,10-tetrathiacyclododecane
CAS.NO:25423-56-7
Synonyms:thiacrown ether {12}aneS4;Tetrathia-12-crown-4;[12]aneS4;MFCD00010829;1,4,7,10-Tetrathiadodecane;1,4,7,10-tetrtathia-cyclododecane;1,4,7,10-TETRATHIACYCLODODECANE
Molecular Formula:C8H16S4
Molecular Weight:240.47300
HS Code:
Physical and Chemical Properties:
Melting point:226-228ºC(lit.)
Boiling point:160-162ºC0.5 mm Hg(lit.)
Density:1.14g/cm3
Index of Refraction:1.573
PSA:101.20000
Exact Mass:240.01300
LogP:2.93280
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:NONH for all modes of transpor
Packing Group:
Contact us for information like thiacrown ether {12}aneS4 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,1,4,7,10-TETRATHIACYCLODODECANE physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,1,4,7,10-TETRATHIACYCLODODECANE Use and application,thiacrown ether {12}aneS4 technical grade,usp/ep/jp grade.
Related News: Steven M. Fruchtman, M.D., President and Chief Executive Officer of Onconova, added ��The rigosertib Pre-approval Access Program is a key strategic initiative for Onconova. 1,4,7,10-tetrathiacyclododecane manufacturer A number of factors influenced the decision by the U.S. Centers for Disease Control and Prevention, including the COVID-19 pandemic, too few facilities to quarantine dogs safely and three recent incidents where rabies-infected dogs were brought into the country, CNN reported. 1,4,7,10-tetrathiacyclododecane supplier Steven M. Fruchtman, M.D., President and Chief Executive Officer of Onconova, added ��The rigosertib Pre-approval Access Program is a key strategic initiative for Onconova. 1,4,7,10-tetrathiacyclododecane vendor The rigosertib Pre-approval Access Program is expected to launch in first half of 2020 and will allow Inceptua to supply intravenous rigosertib within designated countries, primarily and initially concentrated in selected countries in Europe, in response to physician requests for patients with higher-risk MDS who have exhausted all available treatment options, and are not eligible for or have no access to the INSPIRE study. 1,4,7,10-tetrathiacyclododecane factory Steven M. Fruchtman, M.D., President and Chief Executive Officer of Onconova, added ��The rigosertib Pre-approval Access Program is a key strategic initiative for Onconova.

