We serve Chemical Name:diazinane CAS:505-19-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
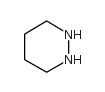
Chemical Name:diazinane
CAS.NO:505-19-1
Synonyms:HEXAHYDROPYRIDAZIN;dihydro-pyridazine;perhydropyridazine;1,2,3,4,5,6-hexahydropyridazine;Hexahydropyridazine;1,2-diazacyclohexane;tetra-hydropyridazine;perhydrodiazine;Pyridazine,hexahydro
Molecular Formula:C4H10N2
Molecular Weight:86.13560
HS Code:2933990090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:107ºC at 760mmHg
Density:0.874g/cm3
Index of Refraction:1.424
PSA:24.06000
Exact Mass:86.08440
LogP:0.53200
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like HEXAHYDROPYRIDAZIN chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Pyridazine,hexahydro physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,1,2,3,4,5,6-hexahydropyridazine Use and application,dihydro-pyridazine technical grade,usp/ep/jp grade.
Related News: On April 8, a group of employees filed an anonymous complaint internally alleging that an executive at its Branchburg, New Jersey, factory had altered documents required by the U.S. Food and Drug Administration. diazinane manufacturer The results of analysis perfectly align with the tau hypothesis — simply put, if the patient is tau biomarker positive, then tau pathology is responsible for his/her cognitive decline, and halting tau pathology should slow or halt progression,” Novak said. “If the patient is negative for markers of tau pathology, then this patient’s impairment is mainly due to other pathologies, and treating tau pathology in this patient won’t be meaningful. diazinane supplier On April 8, a group of employees filed an anonymous complaint internally alleging that an executive at its Branchburg, New Jersey, factory had altered documents required by the U.S. Food and Drug Administration. diazinane vendor After this long manufacturing process, it is purified until it reaches a very high degree of purity and finally becomes an API. diazinane factory On April 8, a group of employees filed an anonymous complaint internally alleging that an executive at its Branchburg, New Jersey, factory had altered documents required by the U.S. Food and Drug Administration.

