We serve Chemical Name:Benzoylecgonine-d3 solution CAS:115732-68-8 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
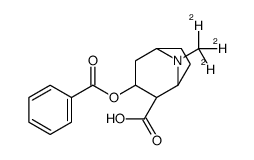
Chemical Name:Benzoylecgonine-d3 solution
CAS.NO:115732-68-8
Synonyms:Benzoylecgonine-N-methyl-d3;3-benzoyloxy-8-(trideuteriomethyl)-8-azabicyclo[3.2.1]octane-4-ca rboxylic acid
Molecular Formula:C16H16D3NO4
Molecular Weight:292.34500
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:66.84000
Exact Mass:292.15000
LogP:1.71720
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:UN 1230 3/PG 2
Packing Group:
Contact us for information like Benzoylecgonine-N-methyl-d3 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,3-benzoyloxy-8-(trideuteriomethyl)-8-azabicyclo[3.2.1]octane-4-ca rboxylic acid physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Benzoylecgonine-N-methyl-d3 Use and application,3-benzoyloxy-8-(trideuteriomethyl)-8-azabicyclo[3.2.1]octane-4-ca rboxylic acid technical grade,usp/ep/jp grade.
Related News: A 40-year-old Chinese woman also tested positive. She was already in quarantine as she is related to another patient who contracted the virus. Benzoylecgonine-d3 solution manufacturer The Basser Center launched its initiative Black & BRCA” in 2020 to bring tailored resources and support to the Black community for genetic counseling and testing.
“At a time when Black men and women are more likely to be diagnosed with cancer at later stages when it is less treatable, Black & BRCA seeks to empower people to understand their family health history and take action to prevent cancer from one generation to the next,” Domchek said.
Suffering through a case of COVID-19 unleashed a host of other health problems in hundreds of thousands of Americans participating in the largest study yet of the long-term effects of coronavirus infection.
Tracking the health insurance records of nearly 2 million people who caught the coronavirus last year, researchers found that one month or more after their infection, almost one-quarter of them sought medical treatment for new conditions, The New York Times reported.
The range of both those affected and the symptoms that struck them was wide. The health issues affected all ages, including children. The most common new health problems were pain; breathing difficulties; high cholesterol; malaise and fatigue; and high blood pressure. But symptoms did not stop there: Some suffered intestinal symptoms; migraines; skin problems; heart abnormalities; sleep disorders; and mental health conditions like anxiety and depression.
Post-COVID health problems did not spare those who had not been seriously ill: While nearly half of patients who were hospitalized for COVID-19 experienced subsequent medical issues, so did 27 percent of people who had mild or moderate symptoms and 19 percent of people who said they were asymptomatic.
“One thing that was surprising to us was the large percentage of asymptomatic patients that are in that category of long COVID,” Robin Gelburd, president of the nonprofit FAIR Health, told the Times.
Gelburd said that since asymptomatic people can have post-COVID symptoms, patients and doctors alike should consider the possibility that some health issues may actually be aftereffects of coronavirus infection.
In total, the report found that more than 454,000 people consulted health providers for symptoms 30 days or more after their infection. The analysis was evaluated by an independent academic reviewer but was not formally peer-reviewed, according to FAIR Health.
“The strength of this study is really its size and its ability to look across the range of disease severity in a diversity of age groups,” Dr. Helen Chu, an associate professor of medicine and infectious diseases at the University of Washington’s School of Medicine, told the Times.
The report “drives home the point that long COVID can affect nearly every organ system,” Dr. Ziyad Al-Aly, chief of the research and development service at the VA St. Louis Health Care System, told the Times.
“Some of these manifestations are chronic conditions that will last a lifetime and will forever scar some individuals and families,” added Al-Aly, who authored a large study published in April on lingering symptoms in COVID-19 patients in the Department of Veterans Affairs health system.
In the latest report, the most common issue for which patients sought medical care was pain — including nerve inflammation and aches and pains associated with nerves and muscles. It was reported by more than a fifth of those who reported post-COVID problems. Breathing difficulties, including shortness of breath, were experienced by 3.5 percent of post-COVID patients.
Nearly 3 percent of patients sought treatment for symptoms that were labeled with diagnostic codes for malaise and fatigue, a far-reaching category that could include issues like brain fog and exhaustion that worsens after physical or mental activity, the Times reported.
The database included only people with private health insurance or Medicare Advantage, not those uninsured or covered by Medicare Parts A, B and D, Medicaid or other government health programs. Chu told the Times that people without insurance or with incomes low enough to qualify for Medicaid are often “more likely to have worse outcomes. Benzoylecgonine-d3 solution supplier The Biogen Inc (BIIB.O) drug, Aduhelm, was authorized based on evidence that it can reduce brain plaques, a likely contributor to Alzheimer’s, rather than proof that it slows progression of the lethal mind-wasting disease. Benzoylecgonine-d3 solution vendor This suspension applies to all dogs, including puppies, emotional support dogs, and dogs that traveled out of the United States and are returning from a high-risk country,” the CDC said in a statement.
The agency said dog rescue missions, imports from dog breeders and people bringing in pets will be affected by the decision, CNN reported.
“If these dogs coming from high-risk countries haven’t been properly vaccinated, there is a risk they could bring it into the country,” Dr. Emily Pieracci, a veterinary medical officer in CDC’s Division of Global Migration and Quarantine, told CNN.
“I think it is important to stress that this is a temporary suspension. We recognize that this is not the long-term solution,” she said, with the initial suspension likely to be 12 months.
Eton Pharmaceuticals, Inc (Nasdaq: ETON) today announced that the U.S. Food and Drug Administration (FDA) has approved Rezipres (ephedrine hydrochloride injection) for the treatment of clinically important hypotension occurring in the setting of anesthesia.
“We are excited to see the approval of Rezipres, which is now our second FDA-approved ready-to-use hospital injectable product. We believe ready-to-use injectable products provide a compelling benefit to hospitals and reduce the need for hospitals to rely on unapproved compounded products,” said Sean Brynjelsen, CEO of Eton Pharmaceuticals. “This innovative sulfite-free formulation has been successfully sold in Europe for years, and we are excited to make it available to U.S. patients shortly.”
Eton Pharmaceuticals, Inc. is an innovative pharmaceutical company focused on developing and commercializing treatments for rare diseases. The company currently owns or receives royalties from four FDA-approved products, including ALKINDI® SPRINKLE, Biorphen®, Rezipres®, and Alaway® Preservative Free, and has five additional products that have been submitted to the FDA.
Statements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including statements associated with the expected ability of Eton to undertake certain activities and accomplish certain goals and objectives.
These statements include but are not limited to statements regarding Eton’s business strategy, Eton’s plans to develop and commercialize its product candidates, the safety and efficacy of Eton’s product candidates, Eton’s plans and expected timing with respect to regulatory filings and approvals, and the size and growth potential of the markets for Eton’s product candidates. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as “believes,” “anticipates,” “plans,” “expects,” “intends,” “will,” “goal,” “potential” and similar expressions are intended to identify forward-looking statements.
These forward-looking statements are based upon Eton’s current expectations and involve assumptions that may never materialize or may prove to be incorrect.
Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks associated with the process of discovering, developing and commercializing drugs that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such drugs.
These and other risks concerning Eton’s development programs and financial position are described in additional detail in Eton’s filings with the Securities and Exchange Commission. All forward-looking statements contained in this press release speak only as of the date on which they were made.
Eton undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies were identified in individuals before the first cases of infection were recognized, according to a study published online June 15 in Clinical Infectious Diseases.
Keri N. Althoff, Ph.D., M.P.H., from the Johns Hopkins Bloomberg School of Public Health in Baltimore, and colleagues aimed to identify individuals with SARS-CoV-2 antibodies in the early weeks of the U.S. pandemic among All of Us participants in all 50 U.S. states who provided blood specimens from Jan. 2 to March 18, 2020.
Participants were considered seropositive if they tested positive for SARS-CoV-2 immunoglobulin G antibodies with the Abbott or EUROIMMUN assays in a sequential testing algorithm.
The researchers found that the estimated sensitivity and specificity of Abbott were100 and 99.5 percent, respectively, and the estimated sensitivity and specificity of EUROIMMUN were 90.7 and 99.7 percent, respectively.
For the sequential testing algorithm used in the study, the net sensitivity and specificity were 90.7 and 100.0 percent, respectively. Overall, nine of the 24,079 study participants with blood specimens from Jan. 2 to March 18, 2020, were seropositive; seven of these were seropositive prior to the first confirmed case in the states of Illinois, Massachusetts, Wisconsin, Pennsylvania, and Mississippi.
“This study contributes to the evidence of low-level circulation of SARS-CoV-2 in many states at the start of the U.S. epidemic,” the authors write. “Future pandemic management should carefully consider the impact of epidemiologic links in testing recommendations and reduce testing restrictions as early as possible. Benzoylecgonine-d3 solution factory A 40-year-old Chinese woman also tested positive. She was already in quarantine as she is related to another patient who contracted the virus.
“At a time when Black men and women are more likely to be diagnosed with cancer at later stages when it is less treatable, Black & BRCA seeks to empower people to understand their family health history and take action to prevent cancer from one generation to the next,” Domchek said.
Suffering through a case of COVID-19 unleashed a host of other health problems in hundreds of thousands of Americans participating in the largest study yet of the long-term effects of coronavirus infection.
Tracking the health insurance records of nearly 2 million people who caught the coronavirus last year, researchers found that one month or more after their infection, almost one-quarter of them sought medical treatment for new conditions, The New York Times reported.
The range of both those affected and the symptoms that struck them was wide. The health issues affected all ages, including children. The most common new health problems were pain; breathing difficulties; high cholesterol; malaise and fatigue; and high blood pressure. But symptoms did not stop there: Some suffered intestinal symptoms; migraines; skin problems; heart abnormalities; sleep disorders; and mental health conditions like anxiety and depression.
Post-COVID health problems did not spare those who had not been seriously ill: While nearly half of patients who were hospitalized for COVID-19 experienced subsequent medical issues, so did 27 percent of people who had mild or moderate symptoms and 19 percent of people who said they were asymptomatic.
“One thing that was surprising to us was the large percentage of asymptomatic patients that are in that category of long COVID,” Robin Gelburd, president of the nonprofit FAIR Health, told the Times.
Gelburd said that since asymptomatic people can have post-COVID symptoms, patients and doctors alike should consider the possibility that some health issues may actually be aftereffects of coronavirus infection.
In total, the report found that more than 454,000 people consulted health providers for symptoms 30 days or more after their infection. The analysis was evaluated by an independent academic reviewer but was not formally peer-reviewed, according to FAIR Health.
“The strength of this study is really its size and its ability to look across the range of disease severity in a diversity of age groups,” Dr. Helen Chu, an associate professor of medicine and infectious diseases at the University of Washington’s School of Medicine, told the Times.
The report “drives home the point that long COVID can affect nearly every organ system,” Dr. Ziyad Al-Aly, chief of the research and development service at the VA St. Louis Health Care System, told the Times.
“Some of these manifestations are chronic conditions that will last a lifetime and will forever scar some individuals and families,” added Al-Aly, who authored a large study published in April on lingering symptoms in COVID-19 patients in the Department of Veterans Affairs health system.
In the latest report, the most common issue for which patients sought medical care was pain — including nerve inflammation and aches and pains associated with nerves and muscles. It was reported by more than a fifth of those who reported post-COVID problems. Breathing difficulties, including shortness of breath, were experienced by 3.5 percent of post-COVID patients.
Nearly 3 percent of patients sought treatment for symptoms that were labeled with diagnostic codes for malaise and fatigue, a far-reaching category that could include issues like brain fog and exhaustion that worsens after physical or mental activity, the Times reported.
The database included only people with private health insurance or Medicare Advantage, not those uninsured or covered by Medicare Parts A, B and D, Medicaid or other government health programs. Chu told the Times that people without insurance or with incomes low enough to qualify for Medicaid are often “more likely to have worse outcomes. Benzoylecgonine-d3 solution supplier The Biogen Inc (BIIB.O) drug, Aduhelm, was authorized based on evidence that it can reduce brain plaques, a likely contributor to Alzheimer’s, rather than proof that it slows progression of the lethal mind-wasting disease. Benzoylecgonine-d3 solution vendor This suspension applies to all dogs, including puppies, emotional support dogs, and dogs that traveled out of the United States and are returning from a high-risk country,” the CDC said in a statement.
The agency said dog rescue missions, imports from dog breeders and people bringing in pets will be affected by the decision, CNN reported.
“If these dogs coming from high-risk countries haven’t been properly vaccinated, there is a risk they could bring it into the country,” Dr. Emily Pieracci, a veterinary medical officer in CDC’s Division of Global Migration and Quarantine, told CNN.
“I think it is important to stress that this is a temporary suspension. We recognize that this is not the long-term solution,” she said, with the initial suspension likely to be 12 months.
Eton Pharmaceuticals, Inc (Nasdaq: ETON) today announced that the U.S. Food and Drug Administration (FDA) has approved Rezipres (ephedrine hydrochloride injection) for the treatment of clinically important hypotension occurring in the setting of anesthesia.
“We are excited to see the approval of Rezipres, which is now our second FDA-approved ready-to-use hospital injectable product. We believe ready-to-use injectable products provide a compelling benefit to hospitals and reduce the need for hospitals to rely on unapproved compounded products,” said Sean Brynjelsen, CEO of Eton Pharmaceuticals. “This innovative sulfite-free formulation has been successfully sold in Europe for years, and we are excited to make it available to U.S. patients shortly.”
Eton Pharmaceuticals, Inc. is an innovative pharmaceutical company focused on developing and commercializing treatments for rare diseases. The company currently owns or receives royalties from four FDA-approved products, including ALKINDI® SPRINKLE, Biorphen®, Rezipres®, and Alaway® Preservative Free, and has five additional products that have been submitted to the FDA.
Statements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including statements associated with the expected ability of Eton to undertake certain activities and accomplish certain goals and objectives.
These statements include but are not limited to statements regarding Eton’s business strategy, Eton’s plans to develop and commercialize its product candidates, the safety and efficacy of Eton’s product candidates, Eton’s plans and expected timing with respect to regulatory filings and approvals, and the size and growth potential of the markets for Eton’s product candidates. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as “believes,” “anticipates,” “plans,” “expects,” “intends,” “will,” “goal,” “potential” and similar expressions are intended to identify forward-looking statements.
These forward-looking statements are based upon Eton’s current expectations and involve assumptions that may never materialize or may prove to be incorrect.
Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks associated with the process of discovering, developing and commercializing drugs that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such drugs.
These and other risks concerning Eton’s development programs and financial position are described in additional detail in Eton’s filings with the Securities and Exchange Commission. All forward-looking statements contained in this press release speak only as of the date on which they were made.
Eton undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies were identified in individuals before the first cases of infection were recognized, according to a study published online June 15 in Clinical Infectious Diseases.
Keri N. Althoff, Ph.D., M.P.H., from the Johns Hopkins Bloomberg School of Public Health in Baltimore, and colleagues aimed to identify individuals with SARS-CoV-2 antibodies in the early weeks of the U.S. pandemic among All of Us participants in all 50 U.S. states who provided blood specimens from Jan. 2 to March 18, 2020.
Participants were considered seropositive if they tested positive for SARS-CoV-2 immunoglobulin G antibodies with the Abbott or EUROIMMUN assays in a sequential testing algorithm.
The researchers found that the estimated sensitivity and specificity of Abbott were100 and 99.5 percent, respectively, and the estimated sensitivity and specificity of EUROIMMUN were 90.7 and 99.7 percent, respectively.
For the sequential testing algorithm used in the study, the net sensitivity and specificity were 90.7 and 100.0 percent, respectively. Overall, nine of the 24,079 study participants with blood specimens from Jan. 2 to March 18, 2020, were seropositive; seven of these were seropositive prior to the first confirmed case in the states of Illinois, Massachusetts, Wisconsin, Pennsylvania, and Mississippi.
“This study contributes to the evidence of low-level circulation of SARS-CoV-2 in many states at the start of the U.S. epidemic,” the authors write. “Future pandemic management should carefully consider the impact of epidemiologic links in testing recommendations and reduce testing restrictions as early as possible. Benzoylecgonine-d3 solution factory A 40-year-old Chinese woman also tested positive. She was already in quarantine as she is related to another patient who contracted the virus.

