We serve Chemical Name:p-ethylbenzylchloride CAS:1467-05-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
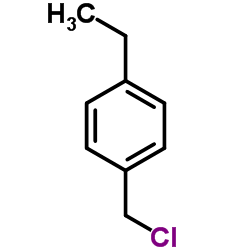
Chemical Name:p-ethylbenzylchloride
CAS.NO:1467-05-6
Synonyms:1-(Chloromethyl)-4-ethylbenzene;Benzene, 1-(chloromethyl)-4-ethyl-;1-(Chlormethyl)-4-ethylbenzol;MFCD00039357;4-Ethylbenzyl chloride;p-ethylbenzylchloride;Toluene, α-chloro-p-ethyl-
Molecular Formula:C9H11Cl
Molecular Weight:154.637
HS Code:2903999090
Physical and Chemical Properties:
Melting point:-21°C
Boiling point:217.1±9.0 °C at 760 mmHg
Density:1.0±0.1 g/cm3
Index of Refraction:1.520
PSA:
Exact Mass:154.054932
LogP:3.48
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:1760
Packing Group:III
Contact us for information like 1-(Chloromethyl)-4-ethylbenzene chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Toluene, α-chloro-p-ethyl- physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,4-Ethylbenzyl chloride Use and application,Benzene, 1-(chloromethyl)-4-ethyl- technical grade,usp/ep/jp grade.
Related News: Patients given the vaccine also experienced a reduction in cerebrospinal fluid biomarkers of abnormal tau, results show. p-ethylbenzylchloride manufacturer There is fierce competition for bulk APIs and overcapacity. However, some high-profit, small-scale drugs still have a good market space. p-ethylbenzylchloride supplier In a statement to Fierce Medtech, Innova said it has completed some corrective actions, while others are still underway, and that the U.S. recall was launched to reclaim tests distributed to employees, clinical studies and to customers for early evaluation. The company said it plans to seek an emergency use authorization and comply with all FDA requirements. p-ethylbenzylchloride vendor In a statement to Fierce Medtech, Innova said it has completed some corrective actions, while others are still underway, and that the U.S. recall was launched to reclaim tests distributed to employees, clinical studies and to customers for early evaluation. The company said it plans to seek an emergency use authorization and comply with all FDA requirements. p-ethylbenzylchloride factory Patients given the vaccine also experienced a reduction in cerebrospinal fluid biomarkers of abnormal tau, results show.

