We serve Chemical Name:4-Isopropyl-5-methylpyrimidine CAS:79644-25-0 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
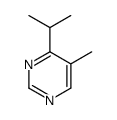
Chemical Name:4-Isopropyl-5-methylpyrimidine
CAS.NO:79644-25-0
Synonyms:4-Isopropyl-5-methylpyrimidine
Molecular Formula:C8H12N2
Molecular Weight:136.19400
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:25.78000
Exact Mass:136.10000
LogP:1.90840
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 4-Isopropyl-5-methylpyrimidine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,4-Isopropyl-5-methylpyrimidine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,4-Isopropyl-5-methylpyrimidine Use and application,4-Isopropyl-5-methylpyrimidine technical grade,usp/ep/jp grade.
Related News: The vaccine also proved safe during the two-year trial, in which eleven doses were administered to randomly chosen patients with mild dementia. People who received the vaccine, known as AADvac1, experienced about the same numbers of side effects and adverse events as those who were given a placebo. 4-Isopropyl-5-methylpyrimidine manufacturer The unit of the British drugmaker, which is challenging HIV drug market leader Gilead Sciences, said it will work with the FDA to determine the next steps for the new drug application. 4-Isopropyl-5-methylpyrimidine supplier Beta Bionics, Inc. recently announced it has received Breakthrough Device designation from the US FDA for its investigational iLet Bionic Pancreas System. 4-Isopropyl-5-methylpyrimidine vendor Aduhelm, however, is in a different league in terms of the number of potential patients and cost to the healthcare system. 4-Isopropyl-5-methylpyrimidine factory INSPIRE is a global, multi-center, randomized, controlled study to assess the efficacy and safety of IV rigosertib in higher-risk MDS (HR-MDS) patients who had progressed on, failed to respond to, or relapsed after previous treatment with a hypomethylating agent (HMA) within nine cycles over the course of one year after initiation of HMA treatment.

