We serve Chemical Name:2-Methyl-4-phenyl-3-butyn-2-ol CAS:1719-19-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
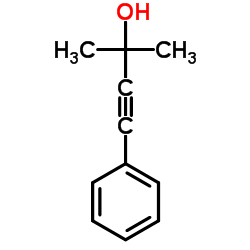
Chemical Name:2-Methyl-4-phenyl-3-butyn-2-ol
CAS.NO:1719-19-3
Synonyms:3-Butyn-2-ol, 2-methyl-4-phenyl-;2-Methyl-4-phenyl-3-butyn-2-ol;2-methyl-4-phenylbut-3-yn-2-ol
Molecular Formula:C11H12O
Molecular Weight:160.212
HS Code:2906299090
Physical and Chemical Properties:
Melting point:48-49℃
Boiling point:259.9±23.0 °C at 760 mmHg
Density:1.0±0.1 g/cm3
Index of Refraction:1.557
PSA:20.23000
Exact Mass:160.088821
LogP:3.02
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 3-Butyn-2-ol, 2-methyl-4-phenyl- chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-methyl-4-phenylbut-3-yn-2-ol physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-methyl-4-phenylbut-3-yn-2-ol Use and application,2-Methyl-4-phenyl-3-butyn-2-ol technical grade,usp/ep/jp grade.
Related News: The CRL for the Cabenuva injection, containing two active ingredients cabotegravir and Janssen��s rilpivirine, follows U.S. market approval in April for its once-a-day pill Dovato, also a two-drug combination. 2-Methyl-4-phenyl-3-butyn-2-ol manufacturer The program caused the price of some drugs to plunge more than 90% when it was introduced last year in some cities, state news agency Xinhua said. 2-Methyl-4-phenyl-3-butyn-2-ol supplier The researchers also found that Black women are much less likely to undergo genetic counseling and testing, largely due to differences in physician recommendations or access to care. 2-Methyl-4-phenyl-3-butyn-2-ol vendor The CRL for the Cabenuva injection, containing two active ingredients cabotegravir and Janssen��s rilpivirine, follows U.S. market approval in April for its once-a-day pill Dovato, also a two-drug combination. 2-Methyl-4-phenyl-3-butyn-2-ol factory In April 2019, Glenmark had received approval from the Drugs Controller General of India (DCGI) for Remogliflozin Etabonate after successfully completing Phase-3 clinical trials.

