We serve Chemical Name:acridin-9-amine,hydrate CAS:65944-23-2 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
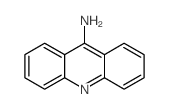
Chemical Name:acridin-9-amine,hydrate
CAS.NO:65944-23-2
Synonyms:9-Acridinamine,hydrate (2:1);MFCD00150520;Acridin-9-amine Hydrate
Molecular Formula:C13H10N2
Molecular Weight:194.23200
HS Code:
Physical and Chemical Properties:
Melting point:230-235ºC
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:38.91000
Exact Mass:194.08400
LogP:3.55140
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 9-Acridinamine,hydrate (2:1) chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Acridin-9-amine Hydrate physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Acridin-9-amine Hydrate Use and application,MFCD00150520 technical grade,usp/ep/jp grade.
Related News: Alira is one of many in the CRO space to ink deals in recent months. Altasciences acquired preclinical CRO Calvert Laboratories in May, weeks after itself being bought out by Novo Holdings, and a few months after buying early-stage CRO WCCT Global. acridin-9-amine,hydrate manufacturer Alira is one of many in the CRO space to ink deals in recent months. Altasciences acquired preclinical CRO Calvert Laboratories in May, weeks after itself being bought out by Novo Holdings, and a few months after buying early-stage CRO WCCT Global. acridin-9-amine,hydrate supplier The foundational patent, which expires in 2034, is owned by MSK and is licensed exclusively to Fate Therapeutics for all human therapeutic uses. acridin-9-amine,hydrate vendor The foundational patent, which expires in 2034, is owned by MSK and is licensed exclusively to Fate Therapeutics for all human therapeutic uses. acridin-9-amine,hydrate factory AbbVie and Johnson & Johnson already boast three FDA-approved regimens for Imbruvica in newly diagnosed chronic lymphocytic leukemia (CLL). Now, the pair aims to add another to the drug’s label, one that could shorten the time patients need to stay on treatment.

