We serve Chemical Name:benzenesulfonylformonitrile CAS:24224-99-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
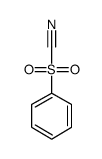
Chemical Name:benzenesulfonylformonitrile
CAS.NO:24224-99-5
Synonyms:benzenesulfonylcyanide;Benzolsulfonyl-cyanid;phenyl sulfonylcyanide
Molecular Formula:C7H5NO2S
Molecular Weight:167.18500
HS Code:2926909090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:271.5ºC at 760 mmHg
Density:1.274 g/mL at 20ºC(lit.)
Index of Refraction:n20/D 1.531
PSA:66.31000
Exact Mass:167.00400
LogP:2.02218
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:UN 2810 6.1/PG 3
Packing Group:
Contact us for information like benzenesulfonylcyanide chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,phenyl sulfonylcyanide physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Benzolsulfonyl-cyanid Use and application,benzenesulfonylcyanide technical grade,usp/ep/jp grade.
Related News: We are pleased to work with Inceptua, given their strong record of administering such programs successfully.�� benzenesulfonylformonitrile manufacturer Exceeding impurities in the drug substance may cause the product and its preparation to be recalled. The company receives an FDA warning letter or a CEP certificate suspension, which in turn will cause customer compensation, product recall costs, and asset impairment losses to affect the company’s performance. benzenesulfonylformonitrile supplier China has asked the European Union for help in buying urgently needed medical supplies from its member countries, the China��s official Xinhua news agency said on Saturday. benzenesulfonylformonitrile vendor The first is the API �C which is the central ingredient. benzenesulfonylformonitrile factory Exceeding impurities in the drug substance may cause the product and its preparation to be recalled. The company receives an FDA warning letter or a CEP certificate suspension, which in turn will cause customer compensation, product recall costs, and asset impairment losses to affect the company’s performance.

