We serve 1-bromo-4-iodobutane CAS:89044-65-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
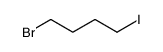
Contact us for information like 1-bromo-4-iodobutane chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,1-Iod-4-brom-butan physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,1-bromo-4-iodobutane Use and application,Butane,1-bromo-4-iodo technical grade,usp/ep/jp grade.
Related News: People should also avoid having visitors, although it is okay for family and friends to drop off parcels or medicines, for example.1-Bromo-2-(trifluoromethoxy)benzene manufacturer Huahai started with the development of characteristic APIs and pharmaceutical intermediates. At the same time, using the international cooperation platform and the opportunity of the expiry of patent protection of major international original research drugs, Huahai has extended the industrial chain to downstream high value-added preparations, and formed intermediates and materials The complete industrial chain of medicine and preparation integration.2-Bromo-9,10-diphenylanthracene supplier Biogen last month revived its plans to seek U.S. approval for its aducanumab treatment after announcing in March that it would terminate two large clinical trials for the drug. But some analysts believed FDA approval is highly unlikely.1,9-Dibromononane vendor Biogen last month revived its plans to seek U.S. approval for its aducanumab treatment after announcing in March that it would terminate two large clinical trials for the drug. But some analysts believed FDA approval is highly unlikely.Coronaviruses (CoV) are a large family of viruses that cause illness ranging from the common cold to more severe diseases such as Middle East Respiratory Syndrome (MERS-CoV) and Severe Acute Respiratory Syndrome (SARS-CoV). A novel coronavirus (nCoV) is a new strain that has not been previously identified in humans.

