We serve Chemical Name:6-amino-2-(2-oxo-2-piperidin-1-ylethyl)sulfanyl-1H-pyrimidin-4-one CAS:36162-27-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
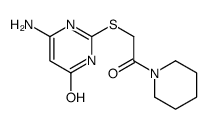
Chemical Name:6-amino-2-(2-oxo-2-piperidin-1-ylethyl)sulfanyl-1H-pyrimidin-4-one
CAS.NO:36162-27-3
Synonyms:2-(2-oxo-2-(piperidin-1-yl)ethylthio)-6-aminopyrimidin-4(1h)-one
Molecular Formula:C11H16N4O2S
Molecular Weight:268.33500
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:117.64000
Exact Mass:268.09900
LogP:1.38810
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 2-(2-oxo-2-(piperidin-1-yl)ethylthio)-6-aminopyrimidin-4(1h)-one chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-(2-oxo-2-(piperidin-1-yl)ethylthio)-6-aminopyrimidin-4(1h)-one physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-(2-oxo-2-(piperidin-1-yl)ethylthio)-6-aminopyrimidin-4(1h)-one Use and application,2-(2-oxo-2-(piperidin-1-yl)ethylthio)-6-aminopyrimidin-4(1h)-one technical grade,usp/ep/jp grade.
Related News: ��With early evidence of clinical activity for our off-the-shelf, iPSC-derived NK cell programs, we are excited to lead in bringing next-generation CAR T-cell therapies to patients and plan to submit an IND for FT819 in the first half of 2020.�� 6-amino-2-(2-oxo-2-piperidin-1-ylethyl)sulfanyl-1H-pyrimidin-4-one manufacturer The American Heart Association explains that metabolic syndrome — a grouping of five different conditions — elevates the risk for such illnesses. Abdominal obesity is one such condition; the other four include high blood sugar, high triglycerides, high blood pressure and low levels of good” HDL cholesterol.
Bariatric surgery — including sleeve gastrectomy and gastric bypass — offers an opportunity to reduce such risk by helping patients achieve considerable weight loss, the investigators said.
In fact, the study team noted that bariatric surgery is the standard of care for severely obese patients. Severe obesity is defined as having a body mass index (BMI) of 40, or a BMI of 35 and up alongside obesity-related complications such as diabetes.
Using insurance claims data, Schimpke and his team focused on a pool of nearly 1.8 million patients across the United States who were severely obese — and therefore eligible for bariatric surgery — in the decade beginning 2010.
Of those, roughly 100,000 actually underwent bariatric surgery during that time frame. But procedure patterns varied widely by state.
For example, while between roughly 9% and 10.4% of eligible patients in New Jersey, Rhode Island and Delaware opted for surgery, less than 3% did so in West Virginia, Alabama and Arkansas.
Overall, the researchers determined that the lowest in opt-in rates by region was the Midwest, where just over 4% of eligible patients underwent surgery, despite the fact that nearly 34% of Midwesterners are obese (making the region home to the highest overall obesity rates in the country).
By contrast, the highest opt-in surgery rate (nearly 8%) was seen in the Northeast region, where the overall obesity rate is lower (29%).
The findings were presented last week at a virtual meeting of the American Society for Metabolic and Bariatric Surgery. Such research is considered preliminary until published in a peer-reviewed journal.
“There are likely several contributing factors to the wide variation in utilization,” said Schimpke. He highlighted differences in: levels of access to medical care; beliefs and attitudes among patients and referring physicians; number of available hospitals and surgeons; and insurance coverage requirements.
Schimpke also pointed to the “negative psycho-social connotation associated with bariatric surgery among both physicians/practitioners and patients, which needs to be addressed with strategic campaigns detailing the safety and efficacy of bariatric surgery. 6-amino-2-(2-oxo-2-piperidin-1-ylethyl)sulfanyl-1H-pyrimidin-4-one supplier ��With early evidence of clinical activity for our off-the-shelf, iPSC-derived NK cell programs, we are excited to lead in bringing next-generation CAR T-cell therapies to patients and plan to submit an IND for FT819 in the first half of 2020.�� 6-amino-2-(2-oxo-2-piperidin-1-ylethyl)sulfanyl-1H-pyrimidin-4-one vendor A November 2020 analysis by researchers at the University of Oxford found Innova’s tests could be more or less accurate depending on who was using them—ranging from lab scientists to trained healthcare professionals to the general public—but determined the tests overall had a low failure rate and high specificity, despite some variations seen in production batches. Still, Innova’s test and antigen diagnostics more broadly have been seen as less accurate compared to gold-standard, lab-based PCR screening. 6-amino-2-(2-oxo-2-piperidin-1-ylethyl)sulfanyl-1H-pyrimidin-4-one factory A November 2020 analysis by researchers at the University of Oxford found Innova’s tests could be more or less accurate depending on who was using them—ranging from lab scientists to trained healthcare professionals to the general public—but determined the tests overall had a low failure rate and high specificity, despite some variations seen in production batches. Still, Innova’s test and antigen diagnostics more broadly have been seen as less accurate compared to gold-standard, lab-based PCR screening.
Bariatric surgery — including sleeve gastrectomy and gastric bypass — offers an opportunity to reduce such risk by helping patients achieve considerable weight loss, the investigators said.
In fact, the study team noted that bariatric surgery is the standard of care for severely obese patients. Severe obesity is defined as having a body mass index (BMI) of 40, or a BMI of 35 and up alongside obesity-related complications such as diabetes.
Using insurance claims data, Schimpke and his team focused on a pool of nearly 1.8 million patients across the United States who were severely obese — and therefore eligible for bariatric surgery — in the decade beginning 2010.
Of those, roughly 100,000 actually underwent bariatric surgery during that time frame. But procedure patterns varied widely by state.
For example, while between roughly 9% and 10.4% of eligible patients in New Jersey, Rhode Island and Delaware opted for surgery, less than 3% did so in West Virginia, Alabama and Arkansas.
Overall, the researchers determined that the lowest in opt-in rates by region was the Midwest, where just over 4% of eligible patients underwent surgery, despite the fact that nearly 34% of Midwesterners are obese (making the region home to the highest overall obesity rates in the country).
By contrast, the highest opt-in surgery rate (nearly 8%) was seen in the Northeast region, where the overall obesity rate is lower (29%).
The findings were presented last week at a virtual meeting of the American Society for Metabolic and Bariatric Surgery. Such research is considered preliminary until published in a peer-reviewed journal.
“There are likely several contributing factors to the wide variation in utilization,” said Schimpke. He highlighted differences in: levels of access to medical care; beliefs and attitudes among patients and referring physicians; number of available hospitals and surgeons; and insurance coverage requirements.
Schimpke also pointed to the “negative psycho-social connotation associated with bariatric surgery among both physicians/practitioners and patients, which needs to be addressed with strategic campaigns detailing the safety and efficacy of bariatric surgery. 6-amino-2-(2-oxo-2-piperidin-1-ylethyl)sulfanyl-1H-pyrimidin-4-one supplier ��With early evidence of clinical activity for our off-the-shelf, iPSC-derived NK cell programs, we are excited to lead in bringing next-generation CAR T-cell therapies to patients and plan to submit an IND for FT819 in the first half of 2020.�� 6-amino-2-(2-oxo-2-piperidin-1-ylethyl)sulfanyl-1H-pyrimidin-4-one vendor A November 2020 analysis by researchers at the University of Oxford found Innova’s tests could be more or less accurate depending on who was using them—ranging from lab scientists to trained healthcare professionals to the general public—but determined the tests overall had a low failure rate and high specificity, despite some variations seen in production batches. Still, Innova’s test and antigen diagnostics more broadly have been seen as less accurate compared to gold-standard, lab-based PCR screening. 6-amino-2-(2-oxo-2-piperidin-1-ylethyl)sulfanyl-1H-pyrimidin-4-one factory A November 2020 analysis by researchers at the University of Oxford found Innova’s tests could be more or less accurate depending on who was using them—ranging from lab scientists to trained healthcare professionals to the general public—but determined the tests overall had a low failure rate and high specificity, despite some variations seen in production batches. Still, Innova’s test and antigen diagnostics more broadly have been seen as less accurate compared to gold-standard, lab-based PCR screening.

