We serve Chemical Name:Devaleryl Valsartan Impurity CAS:676129-92-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
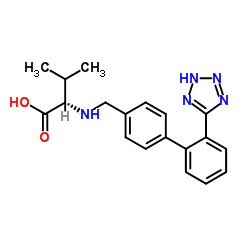
Chemical Name:Devaleryl Valsartan Impurity
CAS.NO:676129-92-3
Synonyms:N-[[2′-(1H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-L-valine;(S)-2-(((2′-(2H-Tetrazol-5-yl)-[1,1′-biphenyl]-4-yl)methyl)amino)-3-methylbutanoic acid;(S)-3-methyl-2-((2′-(1H-tetrazol-5-yl)biphenyl-4-ylmethyl)amino)butyric acid;(S)-3-methyl-2-((2′-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl)-amino)-butyric acid;N-{[2′-(2H-Tetrazol-5-yl)-4-biphenylyl]methyl}-L-valine;L-Valine, N-[[2′-(2H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-;(S)-2-(((2′-(1H-tetrazol-5-yl)-[1,1′-biphenyl]-4-yl)methyl)amino)-3-methylbutanoic acid;Valsartan Impurity 11
Molecular Formula:C19H21N5O2
Molecular Weight:351.402
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:598.2±60.0 °C at 760 mmHg
Density:1.3±0.1 g/cm3
Index of Refraction:1.609
PSA:103.79000
Exact Mass:351.169525
LogP:3.52
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like N-[[2′-(1H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-L-valine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Valsartan Impurity 11 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,(S)-3-methyl-2-((2′-(1H-tetrazol-5-yl)biphenyl-4-ylmethyl)amino)butyric acid Use and application,L-Valine, N-[[2′-(2H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]- technical grade,usp/ep/jp grade.
Related News: The workers are demanding that Hong Kong close all border checkpoints to visitors from mainland China, saying they represent a threat to health care workers in the city. Devaleryl Valsartan Impurity manufacturer After several years of R & D and improvement, the production process of commonly used generic drug bulk drugs is relatively mature, and the products of similar companies have high similarities. Therefore, the competitive advantage of bulk drug companies is mainly reflected in cost control. Companies with cost advantages can usually pass Competition to expand production capacity and further gain scale advantages, while having stable, high-quality upstream supply. Devaleryl Valsartan Impurity supplier The complete chemical and pharmaceutical industry chain consists of basic chemical raw materials, pharmaceutical intermediates, chemical raw materials and chemical preparations. Devaleryl Valsartan Impurity vendor First up is Lilly, which reached all-time highs this week as attention turned to its once highly hyped donanemab, another anti-amyloid that saw a mixed bag of data back in March, with a slight win on one disease scale undermined by a failure on a more widely used measure of Alzheimer’s. Devaleryl Valsartan Impurity factory After several years of R & D and improvement, the production process of commonly used generic drug bulk drugs is relatively mature, and the products of similar companies have high similarities. Therefore, the competitive advantage of bulk drug companies is mainly reflected in cost control. Companies with cost advantages can usually pass Competition to expand production capacity and further gain scale advantages, while having stable, high-quality upstream supply.

