We serve Chemical Name:Dabigatran etexilate CAS:211915-06-9 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
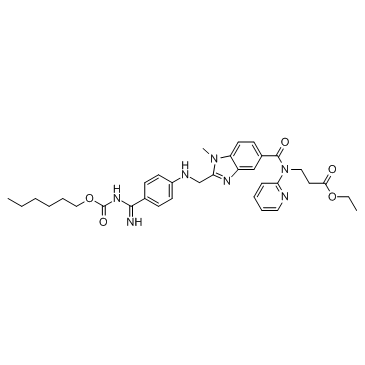
Chemical Name:Dabigatran etexilate
CAS.NO:211915-06-9
Synonyms:BIBR-1048MS;Dabigatranetexilate;Pradaxa;PR sodium salt;Rendix;Dabigatran etexilate;phenolsulfonphthalein sodium salt;Pradax;phenolsulphophthaleine sodium salt;Ethyl N-[(2-{[(4-{N-[(hexyloxy)carbonyl]carbamimidoyl}phenyl)amino]methyl}-1-methyl-1H-benzimidazol-5-yl)carbonyl]-N-2-pyridinyl-β-alaninate;β-Alanine, N-[[2-[[[4-[[[(hexyloxy)carbonyl]amino]iminomethyl]phenyl]amino]methyl]-1-methyl-1H-benzimidazol-5-yl]carbonyl]-N-2-pyridinyl-, ethyl ester;phenolsulfonephthalein sodium;phenol red sodium salt;PHENOL RED,ACS;BIBR-1048
Molecular Formula:C34H41N7O5
Molecular Weight:627.733
HS Code:2933990090
Physical and Chemical Properties:
Melting point:128-129°
Boiling point:827.9±75.0 °C at 760 mmHg
Density:1.2±0.1 g/cm3
Index of Refraction:1.615
PSA:154.03000
Exact Mass:627.316895
LogP:5.13
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like BIBR-1048MS chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,BIBR-1048 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Dabigatranetexilate Use and application,Rendix technical grade,usp/ep/jp grade.
Related News: What’s more, also at the EHA meeting, BeiGene’s Brukinsa showed it could outperform Imbruvica at triggering tumor responses while causing fewer cases of atrial fibrillation, a potentially dangerous heart side effect, in its own head-to-head phase 3 study in previously treated CLL. Dabigatran etexilate manufacturer Onconova is currently in the clinical development stage with oral and IV rigosertib, including clinical trials studying single agent IV rigosertib in second-line higher-risk MDS patients (pivotal Phase 3 INSPIRE trial) and oral rigosertib plus azacitidine in first-line and refractory higher-risk MDS patients (Phase 2). Dabigatran etexilate supplier Onconova is currently in the clinical development stage with oral and IV rigosertib, including clinical trials studying single agent IV rigosertib in second-line higher-risk MDS patients (pivotal Phase 3 INSPIRE trial) and oral rigosertib plus azacitidine in first-line and refractory higher-risk MDS patients (Phase 2). Dabigatran etexilate vendor What’s more, also at the EHA meeting, BeiGene’s Brukinsa showed it could outperform Imbruvica at triggering tumor responses while causing fewer cases of atrial fibrillation, a potentially dangerous heart side effect, in its own head-to-head phase 3 study in previously treated CLL. Dabigatran etexilate factory The New York Times said that the batches being discarded amount to around 60 million doses, citing people familiar with the matter.

