We serve 2-Bromo-4′-chloroacetophenone CAS:536-38-9 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
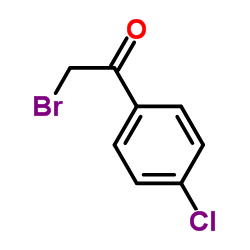
Contact us for information like 2-Bromo-4′-chloroacetophenone chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,4-Chlorophenacyl Bromide physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-Bromo-1-(4-chlorophenyl)ethanone Use and application,2-Bromo-1-(4-chlorophenyl)ethanone technical grade,usp/ep/jp grade.
Related News: According to statistics from the Price Supervision and Competition Bureau of the National Development and Reform Commission, China can produce more than 1,500 kinds of bulk drugs, with a total output of one million tons and an export volume of more than 60%. It has become the world’s second largest drug substance after the United States. Country of manufacture and largest exporter.6-chloro-1-hydroxybenzotriazole manufacturer According to statistics from the Price Supervision and Competition Bureau of the National Development and Reform Commission, China can produce more than 1,500 kinds of bulk drugs, with a total output of one million tons and an export volume of more than 60%. It has become the world’s second largest drug substance after the United States. Country of manufacture and largest exporter.2-Cyano-3-Nitropyridine supplier In the first round of nationwide implement of the program, in September, multinationals including Sanofi and Eli Lilly managed to cut some prices low enough to levels close to those offered by local generic makers.2,3,4,5,6-Pentafluorobenzoyl chloride vendor There is fierce competition for bulk APIs and overcapacity. However, some high-profit, small-scale drugs still have a good market space.Pre-approval Access Programs (also known as expanded access, early access, compassionate use, named patient supply) are regulatory-compliant processes permitting experimental agents in development to be made available upon the request of a physician or a patient for appropriate patients for whom no alternative treatment option exists in their country.

