We serve (4-pyren-1-ylphenyl)boronic acid CAS:872050-52-7 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
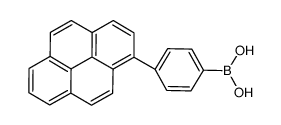
Contact us for information like (4-pyren-1-ylphenyl)boronic acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,4-pyrenylbenzeneboronic acid physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,4-pyrenylbenzeneboronic acid Use and application,(4-pyren-1-ylphenyl)boronic acid technical grade,usp/ep/jp grade.
Related News: Self-isolation involves not going to work, school or into public places.7-chloro-N-(3-chloro-4-fluorophenyl)-6-nitroquinazolin-4-amine manufacturer “Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations” (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials.N-[(S)-Ethoxycarbonyl-1-Butyl]-(S)-Alanine supplier “Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations” (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials.ribothymidine vendor “Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations” (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials.Once they have decided how to make the compound, our staff in the production department manufacture a high quantity of APIs using the large reactors in our plant.

