We serve Chemical Name:magnesium trisilicate hydrate CAS:39365-87-2 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
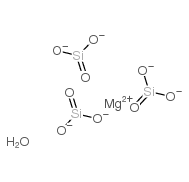
Chemical Name:magnesium trisilicate hydrate
CAS.NO:39365-87-2
Synonyms:MAGNESIUMTRISILICATE,POWDER,USP;Magnesiumtrisilicatex-hydrate;magnesiumtrisilicaten-hydrate;USP)PRS-CODEX
Molecular Formula:H2MgO10Si3—-
Molecular Weight:270.57100
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:100ºC at 760mmHg
Density:N/A
Index of Refraction:
PSA:198.80000
Exact Mass:269.88100
LogP:
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like MAGNESIUMTRISILICATE,POWDER,USP chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,USP)PRS-CODEX physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,USP)PRS-CODEX Use and application,USP)PRS-CODEX technical grade,usp/ep/jp grade.
Related News: If the results from that trial are positive, the company might apply to the U.S. Food and Drug Administration for the same accelerated approval pathway recently used to bring the controversial Alzheimer’s drug aducanumab to market, Novak said. magnesium trisilicate hydrate manufacturer If the results from that trial are positive, the company might apply to the U.S. Food and Drug Administration for the same accelerated approval pathway recently used to bring the controversial Alzheimer’s drug aducanumab to market, Novak said. magnesium trisilicate hydrate supplier Higher-risk MDS is a disease with significant unmet need, and we are pleased to be able to support healthcare professionals seeking access to rigosertib, ahead of its commercial launch,�� said Mark Corbett, EVP, Inceptua Medicines Access. magnesium trisilicate hydrate vendor This leads to low numbers of one or more types of circulating blood cells, and to the need for blood transfusions. In MDS, some of the cells in the bone marrow are abnormal (dysplastic) and may have genetic abnormalities associated with them. magnesium trisilicate hydrate factory If the results from that trial are positive, the company might apply to the U.S. Food and Drug Administration for the same accelerated approval pathway recently used to bring the controversial Alzheimer’s drug aducanumab to market, Novak said.

