We serve Chemical Name:2,7-Dibromo-9-vinyl-9H-carbazole CAS:1438252-33-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
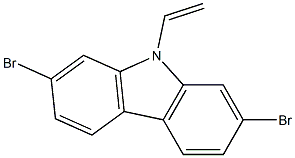
Chemical Name:2,7-Dibromo-9-vinyl-9H-carbazole
CAS.NO:1438252-33-5
Synonyms:2,7-Dibromo-9-vinyl-9H-carbazole
Molecular Formula:C14H9Br2N
Molecular Weight:351.03596
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:
Exact Mass:
LogP:
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 2,7-Dibromo-9-vinyl-9H-carbazole chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2,7-Dibromo-9-vinyl-9H-carbazole physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2,7-Dibromo-9-vinyl-9H-carbazole Use and application,2,7-Dibromo-9-vinyl-9H-carbazole technical grade,usp/ep/jp grade.
Related News: In a statement to Fierce Medtech, Innova said it has completed some corrective actions, while others are still underway, and that the U.S. recall was launched to reclaim tests distributed to employees, clinical studies and to customers for early evaluation. The company said it plans to seek an emergency use authorization and comply with all FDA requirements. 2,7-Dibromo-9-vinyl-9H-carbazole manufacturer In a statement to Fierce Medtech, Innova said it has completed some corrective actions, while others are still underway, and that the U.S. recall was launched to reclaim tests distributed to employees, clinical studies and to customers for early evaluation. The company said it plans to seek an emergency use authorization and comply with all FDA requirements. 2,7-Dibromo-9-vinyl-9H-carbazole supplier Onconova Therapeutics, Inc. and Inceptua Medicines Access (a business unit of the Inceptua Group) recently announced they have entered into a collaboration to make available intravenous rigosertib via a Pre-approval Access Program in selected countries around the world. 2,7-Dibromo-9-vinyl-9H-carbazole vendor In a statement to Fierce Medtech, Innova said it has completed some corrective actions, while others are still underway, and that the U.S. recall was launched to reclaim tests distributed to employees, clinical studies and to customers for early evaluation. The company said it plans to seek an emergency use authorization and comply with all FDA requirements. 2,7-Dibromo-9-vinyl-9H-carbazole factory But several border points remain open, and many medical workers fear that Hong Kong��s well-regarded health care system will be overwhelmed.

