We serve Chemical Name:(2-phenyl-1h-indol-1-yl)acetic acid CAS:62663-25-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
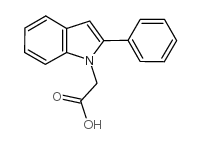
Chemical Name:(2-phenyl-1h-indol-1-yl)acetic acid
CAS.NO:62663-25-6
Synonyms:2-Phenyl-1H-indole-1-acetic acid;2-(2-phenyl-1H-indol-1-yl)acetic acid
Molecular Formula:C16H13NO2
Molecular Weight:251.28000
HS Code:2933990090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:497.9ºC at 760 mmHg
Density:1.19g/cm3
Index of Refraction:
PSA:42.23000
Exact Mass:251.09500
LogP:3.39290
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 2-Phenyl-1H-indole-1-acetic acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-(2-phenyl-1H-indol-1-yl)acetic acid physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-Phenyl-1H-indole-1-acetic acid Use and application,2-Phenyl-1H-indole-1-acetic acid technical grade,usp/ep/jp grade.
Related News: Physiologically relevant interactions with test molecules or protein ligands can be identified with high sensitivity and specificity. (2-phenyl-1h-indol-1-yl)acetic acid manufacturer In October 2020, the Trump administration ordered $375 million worth of Lilly’s COVID-19 antibody therapy bamlanivimab, which is manufactured at the Branchburg plant. (2-phenyl-1h-indol-1-yl)acetic acid supplier Physiologically relevant interactions with test molecules or protein ligands can be identified with high sensitivity and specificity. (2-phenyl-1h-indol-1-yl)acetic acid vendor Physiologically relevant interactions with test molecules or protein ligands can be identified with high sensitivity and specificity. (2-phenyl-1h-indol-1-yl)acetic acid factory In recent years, with the increasing number of patent medicines whose patents have expired, the variety and quantity of generic drugs have also increased rapidly, which has brought huge market opportunities to the API market and the output of APIs has continued to increase.

