We serve Chemical Name:4-(Trifluoromethoxy)benzylamine CAS:93919-56-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
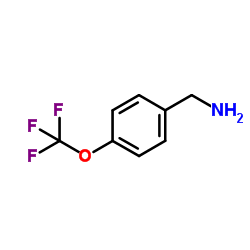
Chemical Name:4-(Trifluoromethoxy)benzylamine
CAS.NO:93919-56-3
Synonyms:Z1R DOXFFF;1-[4-(Trifluoromethoxy)phenyl]methanamine;8200624;EINECS 300-040-1;Benzenemethanamine, 4-(trifluoromethoxy)-;4-(Trifluoromethoxy)benzenemethanamine;4-(Trifluoromethoxy)benzylamine;[4-(Trifluoromethoxy)phenyl]methanamine;p-(Trifluoromethoxy)benzylamine;MFCD00061237
Molecular Formula:C8H8F3NO
Molecular Weight:191.150
HS Code:2922199090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:172.5±0.0 °C at 760 mmHg
Density:1.3±0.1 g/cm3
Index of Refraction:1.471
PSA:35.25000
Exact Mass:191.055801
LogP:2.04
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:UN2735
Packing Group:III
Contact us for information like Z1R DOXFFF chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,MFCD00061237 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,4-(Trifluoromethoxy)benzylamine Use and application,MFCD00061237 technical grade,usp/ep/jp grade.
Related News: At Day 35 following administration, a bone marrow assessment showed that FT819 persisted and continued to demonstrate tumor clearance, whereas primary CAR T cells, while persisting, were not able to control tumor growth. 4-(Trifluoromethoxy)benzylamine manufacturer Onconova Therapeutics, Inc. is a Phase 3-stage biopharmaceutical company focused on discovering and developing novel small molecule drug candidates to treat cancer, with an initial focus on Myelodysplastic Syndromes (MDS). 4-(Trifluoromethoxy)benzylamine supplier The company’s pharmaceutical intermediate business is expected to maintain a growth rate of more than 30%. 4-(Trifluoromethoxy)benzylamine vendor Only when the drug substance is processed into a pharmaceutical preparation can it become a drug for clinical application. 4-(Trifluoromethoxy)benzylamine factory At Day 35 following administration, a bone marrow assessment showed that FT819 persisted and continued to demonstrate tumor clearance, whereas primary CAR T cells, while persisting, were not able to control tumor growth.

