We serve Chemical Name:leucoline CAS:91-22-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
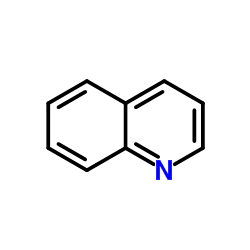
Chemical Name:leucoline
CAS.NO:91-22-5
Synonyms:1-Azanaphthalene;Chinolin;EINECS 202-051-6;1-Benzazine;Quinolin;Chinoline;leucoline;Leucol;β-Quinoline;Quinoline;MFCD00006736;Chinoleine;Benzopyridine;Fasudil Impurity 8
Molecular Formula:C9H7N
Molecular Weight:129.159
HS Code:2933499090
Physical and Chemical Properties:
Melting point:−17-−13 °C(lit.)
Boiling point:234.1±9.0 °C at 760 mmHg
Density:1.1±0.1 g/cm3
Index of Refraction:1.642
PSA:12.89000
Exact Mass:129.057846
LogP:2.08
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:UN 2656 6.1/PG 3
Packing Group:III
Contact us for information like 1-Azanaphthalene chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Fasudil Impurity 8 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,1-Benzazine Use and application,leucoline technical grade,usp/ep/jp grade.
Related News: The policy-oriented emphasis on the medical and pharmaceutical industries and the direct support for the chemical raw material pharmaceutical industry have created a good political environment for the development of related enterprises and laid the foundation for the rapid development of the chemical raw material pharmaceutical industry. The good development prospects of the chemical raw material pharmaceutical industry will be passed directly to the upstream raw material industry, which will help stimulate the demand for the pharmaceutical intermediate market. leucoline manufacturer ��Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations�� (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials. leucoline supplier Many branded versions of drugs are currently more expensive in China than in other major markets. They could now be subjected to a centralized procurement program where manufacturers will have to go through a bidding process to get the right to supply drugs to public hospitals, the National Health Commission said in a document published on late Friday. leucoline vendor The FDA also said that the labeling of the diagnostic, which comes in different versions, included performance claims that did not match up with results seen in clinical studies—and that the data Innova submitted for review “was identical to data previously provided by other manufacturers” in separate requests for emergency COVID authorizations, raising additional questions. leucoline factory The policy-oriented emphasis on the medical and pharmaceutical industries and the direct support for the chemical raw material pharmaceutical industry have created a good political environment for the development of related enterprises and laid the foundation for the rapid development of the chemical raw material pharmaceutical industry. The good development prospects of the chemical raw material pharmaceutical industry will be passed directly to the upstream raw material industry, which will help stimulate the demand for the pharmaceutical intermediate market.

