We serve 1-(sulfamoylamino)propane CAS:147962-41-2 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
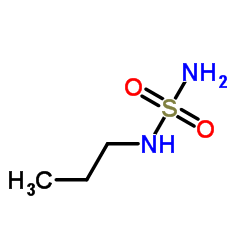
Contact us for information like 1-(sulfamoylamino)propane chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,N-Propylsulfuric diamide physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Propylsulfamide Use and application,1-(sulfamoylamino)propane technical grade,usp/ep/jp grade.
Related News: The API can be directly formulated, and the intermediate can only be used to synthesize the next product. Only through the intermediate can the API be manufactured.1-((4-Aminobenzenemethane)sulfonyl)pyrrolidine manufacturer f you’ve traveled in or transited through mainland China, you won’t be allowed into New Zealand unless you’re a New Zealand national.β-(4-CHLOROPHENYL)GLUTARIC ANHYDRIDE supplier f you’ve traveled in or transited through mainland China, you won’t be allowed into New Zealand unless you’re a New Zealand national.3-bromo-1-phenyl-5-pyridin-2-ylpyridin-2-one vendor Green Valley said it would launch the drug “very soon” in China. The company also aims to roll out a phase-3 clinical trial with sites in the United States, Europe and Asia in early 2020 to facilitate global regulatory approval of the drug.Green Valley said it would launch the drug “very soon” in China. The company also aims to roll out a phase-3 clinical trial with sites in the United States, Europe and Asia in early 2020 to facilitate global regulatory approval of the drug.

