We serve Chemical Name:3-Chloro-2,4-difluorobenzoic acid CAS:154257-75-7 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
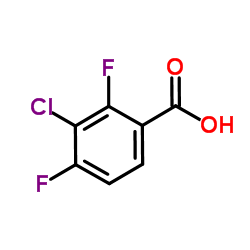
Chemical Name:3-Chloro-2,4-difluorobenzoic acid
CAS.NO:154257-75-7
Synonyms:Benzoic acid, 3-chloro-2,4-difluoro-;QVR CG BF DF;2,4-difluoro-3-chlorobenzoic acid;7290655;3-chloro-2,4-difluoro-benzoic acid 1a;3-chloro-2,4-difluorobenzoicacid;3-Chloro-2,4-difluorobenzoic acid;MFCD01631387
Molecular Formula:C7H3ClF2O2
Molecular Weight:192.547
HS Code:2916399090
Physical and Chemical Properties:
Melting point:174-176ºC
Boiling point:276.7±35.0 °C at 760 mmHg
Density:1.6±0.1 g/cm3
Index of Refraction:1.535
PSA:37.30000
Exact Mass:191.978958
LogP:3.29
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like Benzoic acid, 3-chloro-2,4-difluoro- chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,MFCD01631387 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,3-chloro-2,4-difluoro-benzoic acid 1a Use and application,Benzoic acid, 3-chloro-2,4-difluoro- technical grade,usp/ep/jp grade.
Related News: However, in the long run, as the bulk API market continues to fall, more and more companies will transition to specialty API companies, and competition will intensify in the future. 3-Chloro-2,4-difluorobenzoic acid manufacturer The intravenous form of rigosertib has been studied in Phase 1, 2, and 3 clinical trials involving more than 1000 patients, and is currently being evaluated in a randomized Phase 3 international INSPIRE trial for patients with HR-MDS after failure of HMA therapy. 3-Chloro-2,4-difluorobenzoic acid supplier This time frame optimizes the opportunity to respond to treatment with an HMA prior to declaring treatment failure, as per NCCN Guidelines. Patients are randomized at a 2:1 ratio into two study arms: IV rigosertib plus Best Supportive Care versus Physician��s Choice plus Best Supportive Care. 3-Chloro-2,4-difluorobenzoic acid vendor However, in the long run, as the bulk API market continues to fall, more and more companies will transition to specialty API companies, and competition will intensify in the future. 3-Chloro-2,4-difluorobenzoic acid factory The intravenous form of rigosertib has been studied in Phase 1, 2, and 3 clinical trials involving more than 1000 patients, and is currently being evaluated in a randomized Phase 3 international INSPIRE trial for patients with HR-MDS after failure of HMA therapy.

