We serve Chemical Name:Leflunomide EP Impurity G CAS:724429-16-7 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
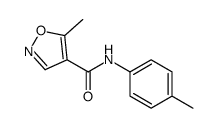
Chemical Name:Leflunomide EP Impurity G
CAS.NO:724429-16-7
Synonyms:unii-46dkd35x8x;Leflunomide EP Impurity G;Leflunomide Impurity 7
Molecular Formula:C12H12N2O2
Molecular Weight:216.23600
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:55.13000
Exact Mass:216.09000
LogP:2.61670
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like unii-46dkd35x8x chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Leflunomide Impurity 7 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,unii-46dkd35x8x Use and application,Leflunomide EP Impurity G technical grade,usp/ep/jp grade.
Related News: Analysts at Mizuho Americas, who spoke to Lilly’s management this week, said that may well have now changed. “Overall, it sounds like the approval raises new questions for Lilly (as it does for many of us!),” the firm said in a note to clients. Leflunomide EP Impurity G manufacturer Onconova Therapeutics, Inc. is a Phase 3-stage biopharmaceutical company focused on discovering and developing novel small molecule drug candidates to treat cancer, with an initial focus on Myelodysplastic Syndromes (MDS). Leflunomide EP Impurity G supplier Dr. Anthony Fauci, director of the U.S. National Institute of Allergy and Infectious Diseases, said, It’s really very impressive,” noting that the vaccine was as good as the most effective shots developed so far during the pandemic. “It’s very important for the world’s population to have, yet again, another highly efficacious vaccine that looks in its trial to have a good safety profile,” Fauci told the Washington Post.
As heartening as the results were, the vaccine may not become a key player in the pandemic until late summer or fall.
Erck told the Post that Novavax will apply for regulatory clearance from a half-dozen countries in the third quarter, which begins in July. With tens of millions of doses already in hand, the company plans to boost manufacturing to produce 100 million doses a month by the end of September and 150 million doses a month in the last three months of the year.
In the United States, the company still needs to file for emergency authorization. The data, which was presented in a news release, will be examined by regulators at the U.S. Food and Drug Administration and by an advisory committee of vaccine advisers. Erck said the vaccine will likely have its biggest initial impact globally, through the World Health Organization’s COVAX initiative.
“A lot of our vaccine is going to be targeted in the early stages for COVAX … and so a lot of those doses are going to get into the low- and middle-income countries first, which is a good thing,” Erck said. Novavax has pledged 1.1 billion doses to COVAX.
The Novavax vaccine was one of six candidates the U.S. government made a huge bet on, investing $1.6 billion to pay for research and development and preordering 110 million doses, the Post reported.
In January, a large U.K. trial showed it was nearly 90% effective, even once a more transmissible variant had taken hold. Over the past five months, health officials and scientists have waited anxiously for confirming evidence from the U.S. trial. But that second study did not start until the end of December, due in part to manufacturing delays.
Meanwhile, the United States had secured more than enough shots from the three companies with authorized vaccines — Pfizer, Moderna and Johnson & Johnson — to satisfy demand. A fourth, from AstraZeneca, reported results in March.
Recombinant protein vaccines such as Novavax’s — the hepatitis B vaccine is another example — teach the immune system to recognize a virus by introducing a lab-made version of a viral protein.
Once the production process is in place, the vaccine offers potential advantages.
“The benefit of their formulation … is it’s remarkably scalable, so they can scale to a very high number of doses,” Matthew Frieman, a coronavirus expert at the University of Maryland’s School of Medicine who has worked with the company in the past, told the Post. “It’s not a super-strange production platform … you don’t need super-specialized facilities. It’s stable, so you don’t need a severe cold chain” to store the vaccine, he said.
The American South and Midwest are home to the highest obesity rates in the nation, but a new study reveals that severely obese residents of those regions are the least likely to choose lifesaving weight-loss surgery.
“Bariatric surgery has been shown to provide long-term weight loss, sustained improvements in cardiovascular and metabolic health, and even prolonged longevity,” noted study author Dr. Scott Schimpke, but the analysis “shows we continue to underutilize the best treatment for morbid obesity and associated metabolic syndrome. Leflunomide EP Impurity G vendor Dr. Anthony Fauci, director of the U.S. National Institute of Allergy and Infectious Diseases, said, It’s really very impressive,” noting that the vaccine was as good as the most effective shots developed so far during the pandemic. “It’s very important for the world’s population to have, yet again, another highly efficacious vaccine that looks in its trial to have a good safety profile,” Fauci told the Washington Post.
As heartening as the results were, the vaccine may not become a key player in the pandemic until late summer or fall.
Erck told the Post that Novavax will apply for regulatory clearance from a half-dozen countries in the third quarter, which begins in July. With tens of millions of doses already in hand, the company plans to boost manufacturing to produce 100 million doses a month by the end of September and 150 million doses a month in the last three months of the year.
In the United States, the company still needs to file for emergency authorization. The data, which was presented in a news release, will be examined by regulators at the U.S. Food and Drug Administration and by an advisory committee of vaccine advisers. Erck said the vaccine will likely have its biggest initial impact globally, through the World Health Organization’s COVAX initiative.
“A lot of our vaccine is going to be targeted in the early stages for COVAX … and so a lot of those doses are going to get into the low- and middle-income countries first, which is a good thing,” Erck said. Novavax has pledged 1.1 billion doses to COVAX.
The Novavax vaccine was one of six candidates the U.S. government made a huge bet on, investing $1.6 billion to pay for research and development and preordering 110 million doses, the Post reported.
In January, a large U.K. trial showed it was nearly 90% effective, even once a more transmissible variant had taken hold. Over the past five months, health officials and scientists have waited anxiously for confirming evidence from the U.S. trial. But that second study did not start until the end of December, due in part to manufacturing delays.
Meanwhile, the United States had secured more than enough shots from the three companies with authorized vaccines — Pfizer, Moderna and Johnson & Johnson — to satisfy demand. A fourth, from AstraZeneca, reported results in March.
Recombinant protein vaccines such as Novavax’s — the hepatitis B vaccine is another example — teach the immune system to recognize a virus by introducing a lab-made version of a viral protein.
Once the production process is in place, the vaccine offers potential advantages.
“The benefit of their formulation … is it’s remarkably scalable, so they can scale to a very high number of doses,” Matthew Frieman, a coronavirus expert at the University of Maryland’s School of Medicine who has worked with the company in the past, told the Post. “It’s not a super-strange production platform … you don’t need super-specialized facilities. It’s stable, so you don’t need a severe cold chain” to store the vaccine, he said.
The American South and Midwest are home to the highest obesity rates in the nation, but a new study reveals that severely obese residents of those regions are the least likely to choose lifesaving weight-loss surgery.
“Bariatric surgery has been shown to provide long-term weight loss, sustained improvements in cardiovascular and metabolic health, and even prolonged longevity,” noted study author Dr. Scott Schimpke, but the analysis “shows we continue to underutilize the best treatment for morbid obesity and associated metabolic syndrome. Leflunomide EP Impurity G factory Russia, which had temporarily stopped issuing work visas to Chinese citizens, is also halting visa-free entry for Chinese tour groups, the government said. Moscow has also stopped issuing electronic tourist visas to individual Chinese travelers.
As heartening as the results were, the vaccine may not become a key player in the pandemic until late summer or fall.
Erck told the Post that Novavax will apply for regulatory clearance from a half-dozen countries in the third quarter, which begins in July. With tens of millions of doses already in hand, the company plans to boost manufacturing to produce 100 million doses a month by the end of September and 150 million doses a month in the last three months of the year.
In the United States, the company still needs to file for emergency authorization. The data, which was presented in a news release, will be examined by regulators at the U.S. Food and Drug Administration and by an advisory committee of vaccine advisers. Erck said the vaccine will likely have its biggest initial impact globally, through the World Health Organization’s COVAX initiative.
“A lot of our vaccine is going to be targeted in the early stages for COVAX … and so a lot of those doses are going to get into the low- and middle-income countries first, which is a good thing,” Erck said. Novavax has pledged 1.1 billion doses to COVAX.
The Novavax vaccine was one of six candidates the U.S. government made a huge bet on, investing $1.6 billion to pay for research and development and preordering 110 million doses, the Post reported.
In January, a large U.K. trial showed it was nearly 90% effective, even once a more transmissible variant had taken hold. Over the past five months, health officials and scientists have waited anxiously for confirming evidence from the U.S. trial. But that second study did not start until the end of December, due in part to manufacturing delays.
Meanwhile, the United States had secured more than enough shots from the three companies with authorized vaccines — Pfizer, Moderna and Johnson & Johnson — to satisfy demand. A fourth, from AstraZeneca, reported results in March.
Recombinant protein vaccines such as Novavax’s — the hepatitis B vaccine is another example — teach the immune system to recognize a virus by introducing a lab-made version of a viral protein.
Once the production process is in place, the vaccine offers potential advantages.
“The benefit of their formulation … is it’s remarkably scalable, so they can scale to a very high number of doses,” Matthew Frieman, a coronavirus expert at the University of Maryland’s School of Medicine who has worked with the company in the past, told the Post. “It’s not a super-strange production platform … you don’t need super-specialized facilities. It’s stable, so you don’t need a severe cold chain” to store the vaccine, he said.
The American South and Midwest are home to the highest obesity rates in the nation, but a new study reveals that severely obese residents of those regions are the least likely to choose lifesaving weight-loss surgery.
“Bariatric surgery has been shown to provide long-term weight loss, sustained improvements in cardiovascular and metabolic health, and even prolonged longevity,” noted study author Dr. Scott Schimpke, but the analysis “shows we continue to underutilize the best treatment for morbid obesity and associated metabolic syndrome. Leflunomide EP Impurity G vendor Dr. Anthony Fauci, director of the U.S. National Institute of Allergy and Infectious Diseases, said, It’s really very impressive,” noting that the vaccine was as good as the most effective shots developed so far during the pandemic. “It’s very important for the world’s population to have, yet again, another highly efficacious vaccine that looks in its trial to have a good safety profile,” Fauci told the Washington Post.
As heartening as the results were, the vaccine may not become a key player in the pandemic until late summer or fall.
Erck told the Post that Novavax will apply for regulatory clearance from a half-dozen countries in the third quarter, which begins in July. With tens of millions of doses already in hand, the company plans to boost manufacturing to produce 100 million doses a month by the end of September and 150 million doses a month in the last three months of the year.
In the United States, the company still needs to file for emergency authorization. The data, which was presented in a news release, will be examined by regulators at the U.S. Food and Drug Administration and by an advisory committee of vaccine advisers. Erck said the vaccine will likely have its biggest initial impact globally, through the World Health Organization’s COVAX initiative.
“A lot of our vaccine is going to be targeted in the early stages for COVAX … and so a lot of those doses are going to get into the low- and middle-income countries first, which is a good thing,” Erck said. Novavax has pledged 1.1 billion doses to COVAX.
The Novavax vaccine was one of six candidates the U.S. government made a huge bet on, investing $1.6 billion to pay for research and development and preordering 110 million doses, the Post reported.
In January, a large U.K. trial showed it was nearly 90% effective, even once a more transmissible variant had taken hold. Over the past five months, health officials and scientists have waited anxiously for confirming evidence from the U.S. trial. But that second study did not start until the end of December, due in part to manufacturing delays.
Meanwhile, the United States had secured more than enough shots from the three companies with authorized vaccines — Pfizer, Moderna and Johnson & Johnson — to satisfy demand. A fourth, from AstraZeneca, reported results in March.
Recombinant protein vaccines such as Novavax’s — the hepatitis B vaccine is another example — teach the immune system to recognize a virus by introducing a lab-made version of a viral protein.
Once the production process is in place, the vaccine offers potential advantages.
“The benefit of their formulation … is it’s remarkably scalable, so they can scale to a very high number of doses,” Matthew Frieman, a coronavirus expert at the University of Maryland’s School of Medicine who has worked with the company in the past, told the Post. “It’s not a super-strange production platform … you don’t need super-specialized facilities. It’s stable, so you don’t need a severe cold chain” to store the vaccine, he said.
The American South and Midwest are home to the highest obesity rates in the nation, but a new study reveals that severely obese residents of those regions are the least likely to choose lifesaving weight-loss surgery.
“Bariatric surgery has been shown to provide long-term weight loss, sustained improvements in cardiovascular and metabolic health, and even prolonged longevity,” noted study author Dr. Scott Schimpke, but the analysis “shows we continue to underutilize the best treatment for morbid obesity and associated metabolic syndrome. Leflunomide EP Impurity G factory Russia, which had temporarily stopped issuing work visas to Chinese citizens, is also halting visa-free entry for Chinese tour groups, the government said. Moscow has also stopped issuing electronic tourist visas to individual Chinese travelers.

