We serve Chemical Name:2,4-dichloro-thiobenzamide CAS:2775-38-4 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
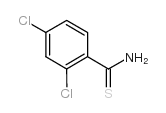
Chemical Name:2,4-dichloro-thiobenzamide
CAS.NO:2775-38-4
Synonyms:2,4,6-TRIBROMOPHENYL ACETATE;2,4-Dichlor-thiobenzamid;2,4-Dichlorothiobenzamide;2,4-Dichlorobenzothioamide;2,4-dichlorobenzene-1-carbothioamide;MFCD00173930
Molecular Formula:C7H5Cl2NS
Molecular Weight:206.09200
HS Code:
Physical and Chemical Properties:
Melting point:135 °C
Boiling point:311.5ºC at 760mmHg
Density:1.473g/cm3
Index of Refraction:1.669
PSA:58.11000
Exact Mass:204.95200
LogP:3.32790
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:UN2811
Packing Group:III
Contact us for information like 2,4,6-TRIBROMOPHENYL ACETATE chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,MFCD00173930 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2,4-Dichlorothiobenzamide Use and application,2,4-dichlorobenzene-1-carbothioamide technical grade,usp/ep/jp grade.
Related News: DMF is the main management method for APIs in developed countries in Europe and the United States. Under the DMF system, API companies can submit DMF filing documents to the regulatory authority at any time, but the regulatory authority will not conduct technical reviews on them. When the drug is administered, the regulatory authority will associate and review the drug substance and the preparation. 2,4-dichloro-thiobenzamide manufacturer With the global industrial division of labor and the change in the business model of multinational pharmaceutical companies, the outsourced market for patented drug substances will further expand. 2,4-dichloro-thiobenzamide supplier This leads to low numbers of one or more types of circulating blood cells, and to the need for blood transfusions. In MDS, some of the cells in the bone marrow are abnormal (dysplastic) and may have genetic abnormalities associated with them. 2,4-dichloro-thiobenzamide vendor DMF is the main management method for APIs in developed countries in Europe and the United States. Under the DMF system, API companies can submit DMF filing documents to the regulatory authority at any time, but the regulatory authority will not conduct technical reviews on them. When the drug is administered, the regulatory authority will associate and review the drug substance and the preparation. 2,4-dichloro-thiobenzamide factory With the global industrial division of labor and the change in the business model of multinational pharmaceutical companies, the outsourced market for patented drug substances will further expand.

