We serve Chemical Name:4,4′-(1,2-Diethylethylene)Diphenol CAS:5635-50-7 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
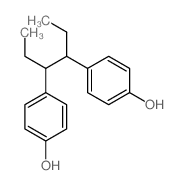
Chemical Name:4,4′-(1,2-Diethylethylene)Diphenol
CAS.NO:5635-50-7
Synonyms:4,4′-(1,2-Diethylethylene)diphenol
Molecular Formula:C18H22O2
Molecular Weight:270.36600
HS Code:
Physical and Chemical Properties:
Melting point:185-188ºC
Boiling point:399.5ºC at 760 mmHg
Density:1.093g/cm3
Index of Refraction:1.582
PSA:40.46000
Exact Mass:270.16200
LogP:4.78520
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 4,4′-(1,2-Diethylethylene)diphenol chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,4,4′-(1,2-Diethylethylene)diphenol physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,4,4′-(1,2-Diethylethylene)diphenol Use and application,4,4′-(1,2-Diethylethylene)diphenol technical grade,usp/ep/jp grade.
Related News: Catalent, a global leader in clinical supply services, recently announced the launch of its new CTSuccessTM clinical trial planning service, designed to support trial sponsors with earlier, more proactive and robust clinical supply chain planning, and reduce the risk of delays and budget overspend common to clinical trials as they progress. 4,4′-(1,2-Diethylethylene)Diphenol manufacturer As part of the agreement, ICIG will enter into a 5-year supply contract to provide Genzyme with materials needed for the production of eliglustat tartrate, an investigational treatment for Gaucher disease Type 1 that is currently in Phase III clinical trials. 4,4′-(1,2-Diethylethylene)Diphenol supplier As part of the agreement, ICIG will enter into a 5-year supply contract to provide Genzyme with materials needed for the production of eliglustat tartrate, an investigational treatment for Gaucher disease Type 1 that is currently in Phase III clinical trials. 4,4′-(1,2-Diethylethylene)Diphenol vendor API (Active Pharmaceutical Ingredient) means the active ingredient which is contained in medicine. 4,4′-(1,2-Diethylethylene)Diphenol factory API (Active Pharmaceutical Ingredient) means the active ingredient which is contained in medicine.

