We serve 5-BROMOPENTYL ACETATE CAS:15848-22-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
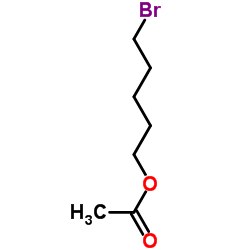
Contact us for information like 5-BROMOPENTYL ACETATE chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,5-Bromo-n-amyl acetate physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,5-Bromo-n-amyl acetate Use and application,5-bromo-1-pentanyl acetate technical grade,usp/ep/jp grade.
Related News: With the improvement of people’s living standards and the aging degree, the demand for medicines has been increasing for a long time. Therefore, the transfer from finished medicines to bulk medicines has greatly promoted the demand for bulk medicines.2-Chloro-1,3-bis(dimentylamino)trimethinium hexafluorophosphate manufacturer With the improvement of people’s living standards and the aging degree, the demand for medicines has been increasing for a long time. Therefore, the transfer from finished medicines to bulk medicines has greatly promoted the demand for bulk medicines.2,6-Difluorobenzyl bromide supplier The clinical trial International Study of Phase 3 IV RigosErtib, or INSPIRE, was finalized following guidance received from the U.S. Food and Drug Administration and European Medicines Agency.2-Aminobenzotrifluoride vendor The clinical trial International Study of Phase 3 IV RigosErtib, or INSPIRE, was finalized following guidance received from the U.S. Food and Drug Administration and European Medicines Agency.The clinical trial International Study of Phase 3 IV RigosErtib, or INSPIRE, was finalized following guidance received from the U.S. Food and Drug Administration and European Medicines Agency.

