We serve Chemical Name:5-chloro-2-(3-methylpiperidin-1-yl)aniline CAS:893751-41-2 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
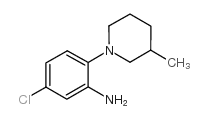
Chemical Name:5-chloro-2-(3-methylpiperidin-1-yl)aniline
CAS.NO:893751-41-2
Synonyms:5-chloro-2-(3-methylpiperidin-1-yl)aniline
Molecular Formula:C12H17ClN2
Molecular Weight:224.73000
HS Code:2933399090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:366.83ºC at 760 mmHg
Density:1.147g/cm3
Index of Refraction:1.577
PSA:29.26000
Exact Mass:224.10800
LogP:3.80470
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 5-chloro-2-(3-methylpiperidin-1-yl)aniline chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,5-chloro-2-(3-methylpiperidin-1-yl)aniline physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,5-chloro-2-(3-methylpiperidin-1-yl)aniline Use and application,5-chloro-2-(3-methylpiperidin-1-yl)aniline technical grade,usp/ep/jp grade.
Related News: Soliris, first approved for generalized myasthenia gravis (gMG) in 2017, carries a list price of about $470,000 annually. But to ICER, that cost is “well beyond typical thresholds.” 1-Imidazolidinecarboxamide,2-imino-3-(4-methoxyphenyl)-N-1-naphthalenyl- manufacturers The Plexxikon patents are for compounds that reduce cancer cell growth by blocking V600E mutated BRAF. Patents were filed as early as 2005. N-((4-butoxyphenyl)carbamothioyl)benzofuran-2-carboxamide suppliers Soliris, first approved for generalized myasthenia gravis (gMG) in 2017, carries a list price of about $470,000 annually. But to ICER, that cost is “well beyond typical thresholds.” cyclobutanecarbonyl iodide vendor & factory Earlier this year, the US Food and Drug Administration approved Verquvo to reduce the risk of cardiovascular death and heart failure hospitalisation, following a hospitalisation for heart failure or need for outpatients intravenous (IV) diuretics in adults with symptomatic chronic heart failure and ejection fraction less than 45%.,The Plexxikon patents are for compounds that reduce cancer cell growth by blocking V600E mutated BRAF. Patents were filed as early as 2005.

