We serve Chemical Name:7-azatryptophan monohydrate CAS:7146-37-4 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
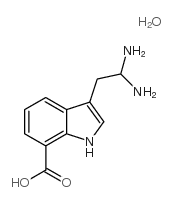
Chemical Name:7-azatryptophan monohydrate
CAS.NO:7146-37-4
Synonyms:A-9690;DL-7-Azatryptophan hydrate
Molecular Formula:C11H15N3O3
Molecular Weight:237.25500
HS Code:
Physical and Chemical Properties:
Melting point:>260ºC (dec.)
Boiling point:548.1ºC at 760 mmHg
Density:N/A
Index of Refraction:
PSA:114.36000
Exact Mass:237.11100
LogP:1.98840
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like A-9690 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,DL-7-Azatryptophan hydrate physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,A-9690 Use and application,A-9690 technical grade,usp/ep/jp grade.
Related News: Additionally, Charles River provides paid time-off for its employees to volunteer for charitable and community organisations. 6-(2,3-dihydroxypropoxy)quinolin-2(1H)-one manufacturers The QbD studies were designed concisely to minimize the use of the API and evaluate the critical product parameters that could affect the product quality attributes of the drug product, per FDA’s QbD guidance. ((2S,3R,6R)-3-acetoxy-1-benzoyl-5-cyano-6-(methoxy-d3)-1,2,3,6-tetrahydropyridin-2-yl)methyl benzoate suppliers The additional GMP labs will be able to develop and manufacture highly potent API (HPAPI) under GMP standards. The expanded labs and manufacturing facilities are expected to come online between 1Q22 and 3Q22. 3,4,5-trihydroxy-benzoic acid isopropylidenehydrazide vendor & factory As currently constituted, Vanamali employs roughly 140 works in Hyderabad, Telangana, that specialize in API starting materials and contract research, according to its website. Wavelength said the Indian firm currently sports enough capacity for research- to commercial-scale production of its drug intermediates.,The QbD studies were designed concisely to minimize the use of the API and evaluate the critical product parameters that could affect the product quality attributes of the drug product, per FDA’s QbD guidance.

