We serve Chemical Name:5-(phenoxymethyl)thiophene-2-carboxylic acid CAS:61855-05-8 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
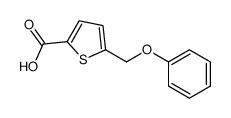
Chemical Name:5-(phenoxymethyl)thiophene-2-carboxylic acid
CAS.NO:61855-05-8
Synonyms:5-phenoxymethyl-thiophene-2-carboxylic acid
Molecular Formula:C12H10O3S
Molecular Weight:234.27100
HS Code:2934999090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:74.77000
Exact Mass:234.03500
LogP:3.02530
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 5-phenoxymethyl-thiophene-2-carboxylic acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,5-phenoxymethyl-thiophene-2-carboxylic acid physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,5-phenoxymethyl-thiophene-2-carboxylic acid Use and application,5-phenoxymethyl-thiophene-2-carboxylic acid technical grade,usp/ep/jp grade.
Related News: These sites operate under cGMP conditions and have been inspected and approved by leading global pharmaceutical companies and regulatory authorities, including the US FDA. 2-(4-Carbomethoxybutyryl)-2-methylcyclohexanone manufacturers Meanwhile, EMA’s Committee for Medicinal Products for Human Use adopted a positive opinion recommending marketing authorization for Moderna’s Covid-19 vaccine, now known as Spikevax, to include adolescents 12 years of age and older. 17β-acetoxy-4-chloroestradiol suppliers EMA said the drug can help improve the respiratory function of Pompe disease patients, and the most common side effects include hypersensitivity (including anaphylaxis) and infusion-associated reactions. 6-(4-(2,2,2-trichloroacetyl)piperazin-1-yl)benzo[d]thiazol-2(3H)-one vendor & factory Meanwhile, EMA’s Committee for Medicinal Products for Human Use adopted a positive opinion recommending marketing authorization for Moderna’s Covid-19 vaccine, now known as Spikevax, to include adolescents 12 years of age and older. ,EMA said the drug can help improve the respiratory function of Pompe disease patients, and the most common side effects include hypersensitivity (including anaphylaxis) and infusion-associated reactions.

