We serve Chemical Name:pivalic acid potassium salt CAS:19455-23-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
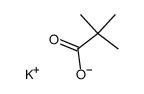
Chemical Name:pivalic acid potassium salt
CAS.NO:19455-23-3
Synonyms:Potassium pivalate;potassium 2,2-dimethylpropionate;trimethylacetic acid potassium salt;Kpivalate;potassium pivalate
Molecular Formula:C5H9KO2
Molecular Weight:140.22200
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:40.13000
Exact Mass:140.02400
LogP:
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like Potassium pivalate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,potassium pivalate physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Kpivalate Use and application,potassium pivalate technical grade,usp/ep/jp grade.
Related News: Grégoire then joined telecoms service provider Cirpack, following its acquisition of Andrexen, as Chief Marketing and Product Officer. He most recently held the position of Chief Strategy Officer at Experty.io, a blockchain-based knowledge sharing platform. tert-Butyl (3-hydrazino-3-oxopropyl)carbamate manufacturers For these reasons, many pharmaceutical companies are choosing to evaluate biocatalytic processes during early-stage drug development to avoid missing opportunities for capitalizing on these gains. 1-Benzyl-7-phenyl-1H-pyrimido[4,5-d][1,3]oxazine-2,4-dione suppliers Moderna filed an application with the FDA for that same younger age group on June 10 but has yet to hear from the agency. States like Rhode Island have already signed off on allowing 12 to 17-year-olds to use the vaccine. Strontium dithionate tetrahydrate vendor & factory Dr. Elsie Melsopp, Head of Solids Formulations for Alcami, says the company successfully helped a client overcome an API supply shortage using Quality by Design (QbD) studies to successfully formulate an orphan drug product. ,For these reasons, many pharmaceutical companies are choosing to evaluate biocatalytic processes during early-stage drug development to avoid missing opportunities for capitalizing on these gains.

