We serve Chemical Name:flucofuron CAS:370-50-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
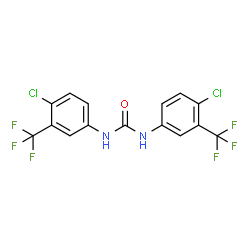
Chemical Name:flucofuron
CAS.NO:370-50-3
Synonyms:N,N’-bis[4-chloro-3-(trifluoromethyl)phenyl]urea;1,3-Bis[4-chloro-3-(trifluoromethyl)phenyl]urea;flucofuron;N,N’-Bis[4-chloro-3-(trifluoromethyl)phenyl]carbamimidic acid;N,N’-Bis[4-chloro-3-(trifluoromethyl)phenyl]urea;tcmdc-137559;1,3-bis(4-chloro-α,α,α-trifluoro-m-tolyl)urea;1,3-Bis(4-chloro-a,a,a-trifluoro-m-tolyl)urea;Methanol, 1-[[4-chloro-3-(trifluoromethyl)phenyl]amino]-1-[[4-chloro-3-(trifluoromethyl)phenyl]imino]-, (E)-;Urea, N,N’-bis[4-chloro-3-(trifluoromethyl)phenyl]-;1,3-Bis[4-chloro-3-(trifluoromethyl)phenyl]urea;EINECS 206-728-7;Sorafenib Impurity I
Molecular Formula:C15H8Cl2F6N2O
Molecular Weight:417.133
HS Code:2924299090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:339.9±42.0 °C at 760 mmHg
Density:1.6±0.1 g/cm3
Index of Refraction:1.564
PSA:41.13000
Exact Mass:415.991791
LogP:7.82
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like N,N’-bis[4-chloro-3-(trifluoromethyl)phenyl]urea chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Sorafenib Impurity I physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Urea, N,N’-bis[4-chloro-3-(trifluoromethyl)phenyl]- Use and application,EINECS 206-728-7 technical grade,usp/ep/jp grade.
Related News: He said it is “normal” for scientists and clinicians to discuss and debate data from experiments and clinical trials, but added those discussions have taken a turn “outside the boundaries of legitimate scientific deliberation.” 2-acetylbicyclo(2.2.1)hepta-2,5-diene manufacturers According to Plexxikon, GSK scientists were only able to make Tafinlar after talks with Plexxikon about a potential partnership; although those talks never turned into a licensing deal, GSK apparently channeled that information and created a rival drug. [[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] [(2R,3S,4R,5R)-5-(3,4-dimethylpyridin-1-ium-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl phosphate suppliers Moderna filed an application with the FDA for that same younger age group on June 10 but has yet to hear from the agency. States like Rhode Island have already signed off on allowing 12 to 17-year-olds to use the vaccine. samarium(III) hexacyanocobaltate(III) hydrate vendor & factory The company was one of many to step into the void for needed generic medicines as chronic shortages crippled access to key drugs, specifically in the early- to mid-stages of the pandemic. That shortage actually led the FDA to thaw its relationship with compounding pharmacies — which was once particularly icy — to ramp up production.,According to Plexxikon, GSK scientists were only able to make Tafinlar after talks with Plexxikon about a potential partnership; although those talks never turned into a licensing deal, GSK apparently channeled that information and created a rival drug.

